 Found 869 hits Enz. Inhib. hit(s) with Target = 'Matrix metalloproteinase-7 (MMP7)'
Found 869 hits Enz. Inhib. hit(s) with Target = 'Matrix metalloproteinase-7 (MMP7)' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Matrilysin
(Homo sapiens (Human)) | BDBM50102594

(7-Isobutyl-8-oxo-2-oxa-9-aza-bicyclo[10.2.2]hexade...)Show SMILES CNC(=O)CNC(=O)[C@@H]1Cc2ccc(OCCC[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO)cc2 Show InChI InChI=1S/C23H34N4O6/c1-14(2)11-18-17(22(30)27-32)5-4-10-33-16-8-6-15(7-9-16)12-19(26-21(18)29)23(31)25-13-20(28)24-3/h6-9,14,17-19,32H,4-5,10-13H2,1-3H3,(H,24,28)(H,25,31)(H,26,29)(H,27,30)/t17-,18+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-7 |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50102608
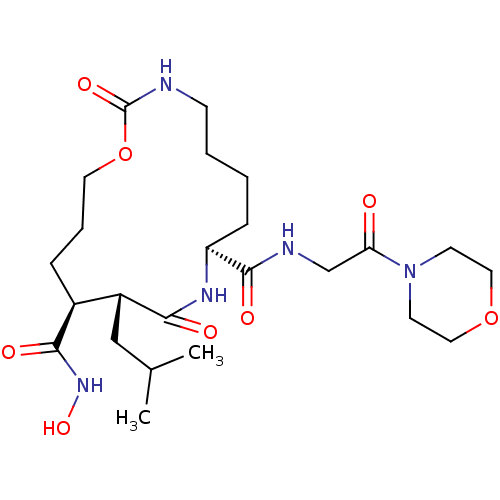
(11-Isobutyl-2,10-dioxo-1-oxa-3,9-diaza-cyclopentad...)Show SMILES CC(C)C[C@@H]1[C@H](CCCOC(=O)NCCCC[C@H](NC1=O)C(=O)NCC(=O)N1CCOCC1)C(=O)NO Show InChI InChI=1S/C24H41N5O8/c1-16(2)14-18-17(22(32)28-35)6-5-11-37-24(34)25-8-4-3-7-19(27-21(18)31)23(33)26-15-20(30)29-9-12-36-13-10-29/h16-19,35H,3-15H2,1-2H3,(H,25,34)(H,26,33)(H,27,31)(H,28,32)/t17-,18+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-7 |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50086884
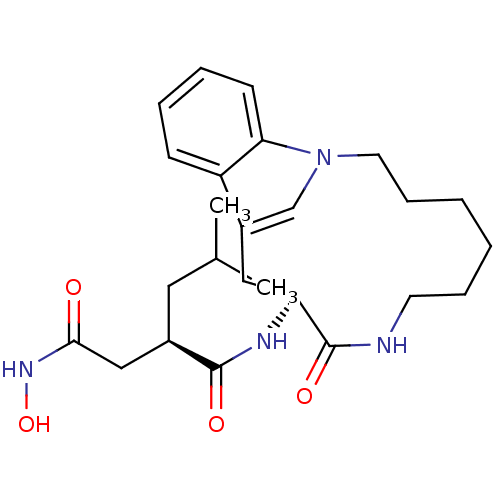
((R)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-9-oxo-1,8-di...)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@H]1Cc2cn(CCCCCCNC1=O)c1ccccc21 Show InChI InChI=1S/C25H36N4O4/c1-17(2)13-18(15-23(30)28-33)24(31)27-21-14-19-16-29(22-10-6-5-9-20(19)22)12-8-4-3-7-11-26-25(21)32/h5-6,9-10,16-18,21,33H,3-4,7-8,11-15H2,1-2H3,(H,26,32)(H,27,31)(H,28,30)/t18-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-7 |
Bioorg Med Chem Lett 5: 1415-1420 (1995)
Article DOI: 10.1016/0960-894X(95)00233-J
BindingDB Entry DOI: 10.7270/Q2Z89CBH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Matrilysin
(Homo sapiens (Human)) | BDBM50078936
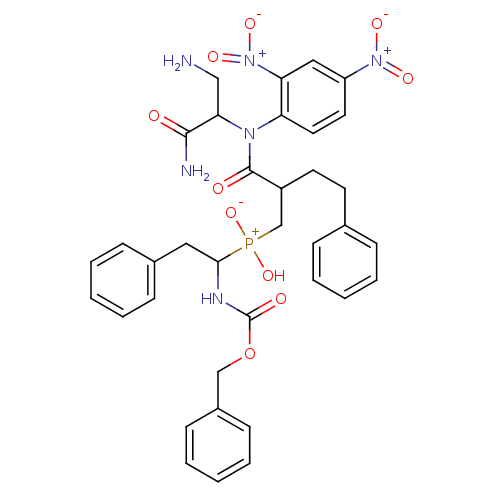
(CHEMBL87786 | {2-[(2-Amino-1-carbamoyl-ethyl)-(2,4...)Show SMILES NCC(N(C(=O)C(CCc1ccccc1)C[P+](O)([O-])C(Cc1ccccc1)NC(=O)OCc1ccccc1)c1ccc(cc1[N+]([O-])=O)[N+]([O-])=O)C(N)=O Show InChI InChI=1S/C36H39N6O10P/c37-22-32(34(38)43)40(30-19-18-29(41(46)47)21-31(30)42(48)49)35(44)28(17-16-25-10-4-1-5-11-25)24-53(50,51)33(20-26-12-6-2-7-13-26)39-36(45)52-23-27-14-8-3-9-15-27/h1-15,18-19,21,28,32-33H,16-17,20,22-24,37H2,(H2,38,43)(H,39,45)(H,50,51) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloprotease-7 Matrilysin |
J Med Chem 42: 2610-20 (1999)
Article DOI: 10.1021/jm9900164
BindingDB Entry DOI: 10.7270/Q22B8X71 |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50284753
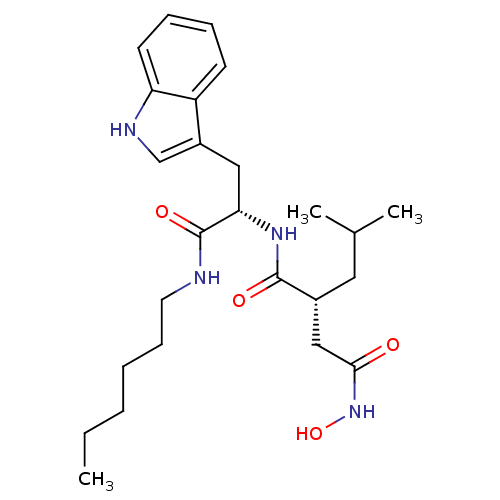
((R)-N*1*-[(S)-1-Hexylcarbamoyl-2-(1H-indol-3-yl)-e...)Show SMILES CCCCCCNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO Show InChI InChI=1S/C25H38N4O4/c1-4-5-6-9-12-26-25(32)22(14-19-16-27-21-11-8-7-10-20(19)21)28-24(31)18(13-17(2)3)15-23(30)29-33/h7-8,10-11,16-18,22,27,33H,4-6,9,12-15H2,1-3H3,(H,26,32)(H,28,31)(H,29,30)/t18-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-7 |
Bioorg Med Chem Lett 5: 1415-1420 (1995)
Article DOI: 10.1016/0960-894X(95)00233-J
BindingDB Entry DOI: 10.7270/Q2Z89CBH |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50229322
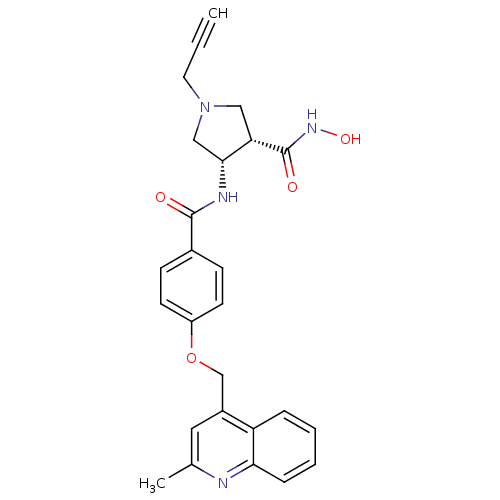
((3S,4S)-N-hydroxy-4-(4-((2-methylquinolin-4-yl)met...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CN(CC#C)C[C@@H]2C(=O)NO)c2ccccc2n1 |r| Show InChI InChI=1S/C26H26N4O4/c1-3-12-30-14-22(26(32)29-33)24(15-30)28-25(31)18-8-10-20(11-9-18)34-16-19-13-17(2)27-23-7-5-4-6-21(19)23/h1,4-11,13,22,24,33H,12,14-16H2,2H3,(H,28,31)(H,29,32)/t22-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to MMP7 |
Bioorg Med Chem Lett 18: 694-9 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.059
BindingDB Entry DOI: 10.7270/Q2BV7HGG |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50433877

(CHEMBL2380390)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](CC(C)C)S[C@H]1CN(C[C@H]1S)C(=O)CCCCCN1C(=O)c2ccccc2C1=O |r| Show InChI InChI=1S/C34H44N4O5S2/c1-22(2)18-28(32(41)36-26(31(40)35-3)19-23-12-6-4-7-13-23)45-29-21-37(20-27(29)44)30(39)16-8-5-11-17-38-33(42)24-14-9-10-15-25(24)34(38)43/h4,6-7,9-10,12-15,22,26-29,44H,5,8,11,16-21H2,1-3H3,(H,35,40)(H,36,41)/t26-,27+,28+,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida State University
Curated by ChEMBL
| Assay Description
Inhibition of MMP-7 (unknown origin) using Mca-PLGLDpa-AR-NH2 as substrate preincubated for 30 mins by fluorescence assay |
J Med Chem 56: 4357-73 (2013)
Article DOI: 10.1021/jm400529f
BindingDB Entry DOI: 10.7270/Q21Z45TX |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50433878
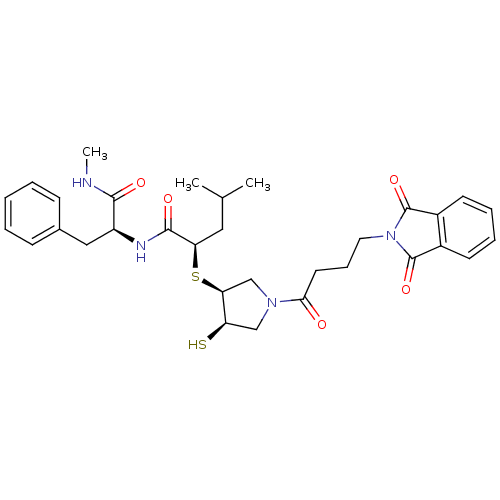
(CHEMBL2380389)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](CC(C)C)S[C@H]1CN(C[C@H]1S)C(=O)CCCN1C(=O)c2ccccc2C1=O |r| Show InChI InChI=1S/C32H40N4O5S2/c1-20(2)16-26(30(39)34-24(29(38)33-3)17-21-10-5-4-6-11-21)43-27-19-35(18-25(27)42)28(37)14-9-15-36-31(40)22-12-7-8-13-23(22)32(36)41/h4-8,10-13,20,24-27,42H,9,14-19H2,1-3H3,(H,33,38)(H,34,39)/t24-,25+,26+,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida State University
Curated by ChEMBL
| Assay Description
Inhibition of MMP-7 (unknown origin) using Mca-PLGLDpa-AR-NH2 as substrate preincubated for 30 mins by fluorescence assay |
J Med Chem 56: 4357-73 (2013)
Article DOI: 10.1021/jm400529f
BindingDB Entry DOI: 10.7270/Q21Z45TX |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50555051
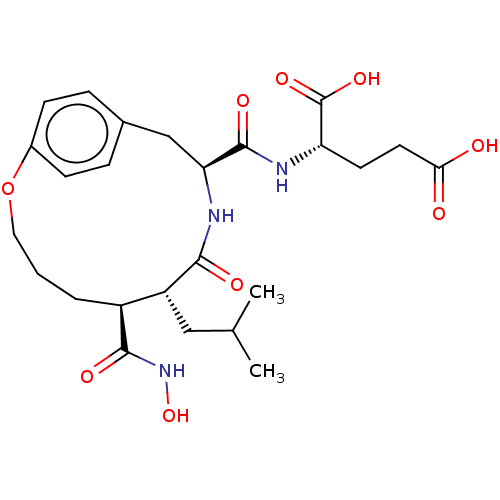
(CHEMBL4739996)Show SMILES CC(C)C[C@@H]1[C@H](CCCOc2ccc(C[C@H](NC1=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cc2)C(=O)NO |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant MMP7 using Mca-Lys-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01514
BindingDB Entry DOI: 10.7270/Q2PC361P |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50555050
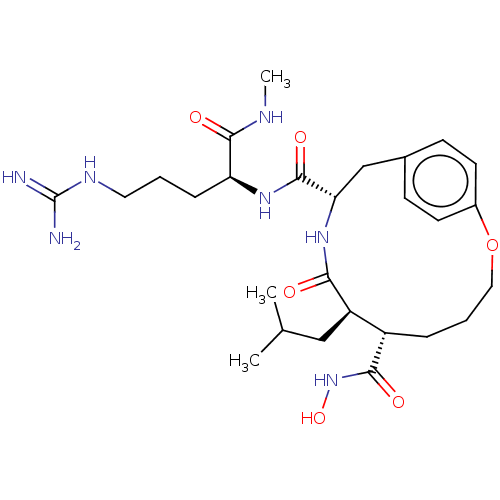
(CHEMBL4764575)Show SMILES CNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1Cc2ccc(OCCC[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO)cc2 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant MMP7 using Mca-Lys-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01514
BindingDB Entry DOI: 10.7270/Q2PC361P |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50183711
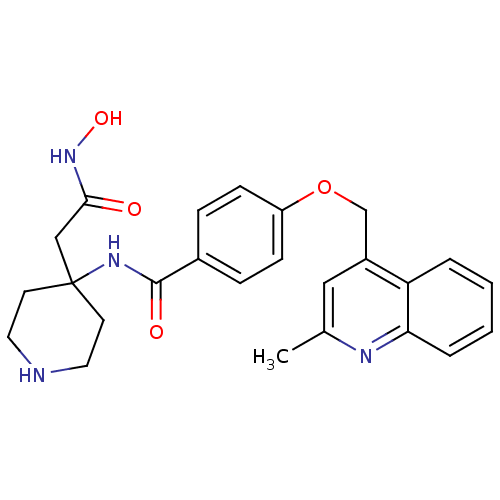
(CHEMBL208009 | N-(4-(2-(hydroxyamino)-2-oxoethyl)p...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NC2(CC(=O)NO)CCNCC2)c2ccccc2n1 Show InChI InChI=1S/C25H28N4O4/c1-17-14-19(21-4-2-3-5-22(21)27-17)16-33-20-8-6-18(7-9-20)24(31)28-25(15-23(30)29-32)10-12-26-13-11-25/h2-9,14,26,32H,10-13,15-16H2,1H3,(H,28,31)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to MMP7 |
Bioorg Med Chem Lett 16: 2699-704 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.015
BindingDB Entry DOI: 10.7270/Q2TB16H3 |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50284755
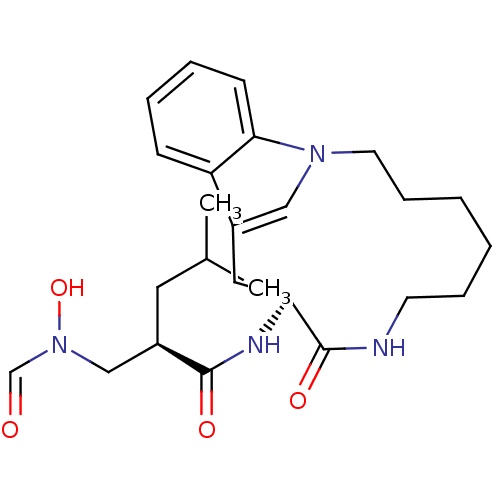
(2-[(Formyl-hydroxy-amino)-methyl]-4-methyl-pentano...)Show SMILES CC(C)C[C@H](CN(O)C=O)C(=O)N[C@H]1Cc2cn(CCCCCCNC1=O)c1ccccc21 Show InChI InChI=1S/C25H36N4O4/c1-18(2)13-20(16-29(33)17-30)24(31)27-22-14-19-15-28(23-10-6-5-9-21(19)23)12-8-4-3-7-11-26-25(22)32/h5-6,9-10,15,17-18,20,22,33H,3-4,7-8,11-14,16H2,1-2H3,(H,26,32)(H,27,31)/t20-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-7 |
Bioorg Med Chem Lett 5: 1415-1420 (1995)
Article DOI: 10.1016/0960-894X(95)00233-J
BindingDB Entry DOI: 10.7270/Q2Z89CBH |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50183715
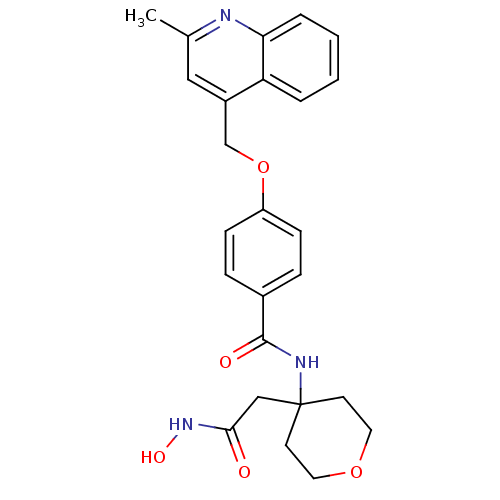
(CHEMBL207305 | N-(4-(2-(hydroxyamino)-2-oxoethyl)-...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NC2(CC(=O)NO)CCOCC2)c2ccccc2n1 Show InChI InChI=1S/C25H27N3O5/c1-17-14-19(21-4-2-3-5-22(21)26-17)16-33-20-8-6-18(7-9-20)24(30)27-25(15-23(29)28-31)10-12-32-13-11-25/h2-9,14,31H,10-13,15-16H2,1H3,(H,27,30)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to MMP7 |
Bioorg Med Chem Lett 16: 2699-704 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.015
BindingDB Entry DOI: 10.7270/Q2TB16H3 |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50183715

(CHEMBL207305 | N-(4-(2-(hydroxyamino)-2-oxoethyl)-...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NC2(CC(=O)NO)CCOCC2)c2ccccc2n1 Show InChI InChI=1S/C25H27N3O5/c1-17-14-19(21-4-2-3-5-22(21)26-17)16-33-20-8-6-18(7-9-20)24(30)27-25(15-23(29)28-31)10-12-32-13-11-25/h2-9,14,31H,10-13,15-16H2,1H3,(H,27,30)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of MMP7 |
Bioorg Med Chem 16: 8781-94 (2008)
Article DOI: 10.1016/j.bmc.2008.08.058
BindingDB Entry DOI: 10.7270/Q2JD4WM2 |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50066659
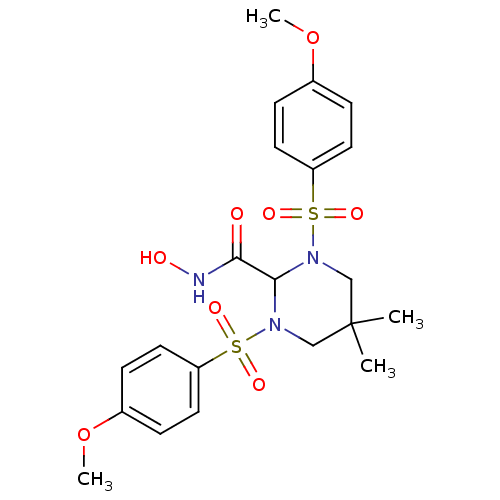
(1,3-BIS-(4-METHOXY-BENZENESULFONYL)-5,5-DIMETHYL-H...)Show SMILES COc1ccc(cc1)S(=O)(=O)N1CC(C)(C)CN(C1C(=O)NO)S(=O)(=O)c1ccc(OC)cc1 Show InChI InChI=1S/C21H27N3O8S2/c1-21(2)13-23(33(27,28)17-9-5-15(31-3)6-10-17)20(19(25)22-26)24(14-21)34(29,30)18-11-7-16(32-4)8-12-18/h5-12,20,26H,13-14H2,1-4H3,(H,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-7 (MMP-7). |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50555052
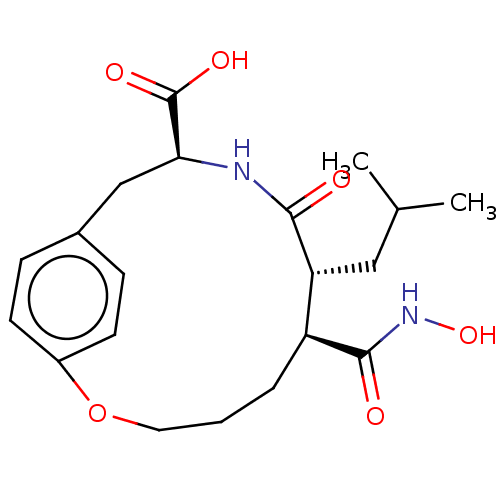
(CHEMBL4751015)Show SMILES CC(C)C[C@@H]1[C@H](CCCOc2ccc(C[C@H](NC1=O)C(O)=O)cc2)C(=O)NO |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant MMP7 using Mca-Lys-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01514
BindingDB Entry DOI: 10.7270/Q2PC361P |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50433879
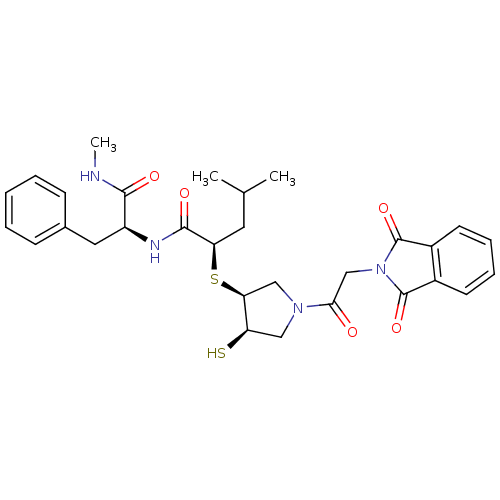
(CHEMBL2380388)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](CC(C)C)S[C@H]1CN(C[C@H]1S)C(=O)CN1C(=O)c2ccccc2C1=O |r| Show InChI InChI=1S/C30H36N4O5S2/c1-18(2)13-24(28(37)32-22(27(36)31-3)14-19-9-5-4-6-10-19)41-25-16-33(15-23(25)40)26(35)17-34-29(38)20-11-7-8-12-21(20)30(34)39/h4-12,18,22-25,40H,13-17H2,1-3H3,(H,31,36)(H,32,37)/t22-,23+,24+,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida State University
Curated by ChEMBL
| Assay Description
Inhibition of MMP-7 (unknown origin) using Mca-PLGLDpa-AR-NH2 as substrate preincubated for 30 mins by fluorescence assay |
J Med Chem 56: 4357-73 (2013)
Article DOI: 10.1021/jm400529f
BindingDB Entry DOI: 10.7270/Q21Z45TX |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50082556

((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(11-12-27-18)28(24,25)15-5-3-13(4-6-15)26-14-7-9-19-10-8-14/h3-10,16,23H,11-12H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of matrilysin (Matrix metalloprotease-7) |
J Med Chem 42: 4547-62 (1999)
BindingDB Entry DOI: 10.7270/Q2D79C32 |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50592895
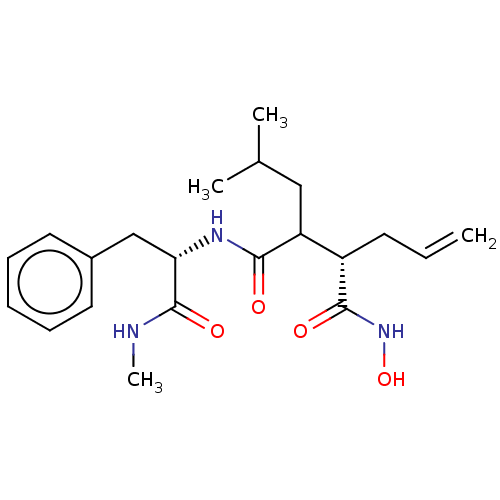
(CHEMBL5187653)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)C(CC(C)C)[C@H](CC=C)C(=O)NO |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01855
BindingDB Entry DOI: 10.7270/Q2GB281X |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50265157
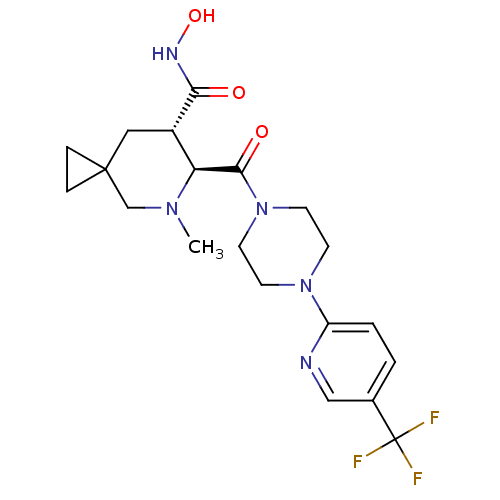
((6S,7S)-N-hydroxy-5-methyl-6-(4-(5-(trifluoromethy...)Show SMILES CN1CC2(CC2)C[C@@H]([C@H]1C(=O)N1CCN(CC1)c1ccc(cn1)C(F)(F)F)C(=O)NO |r| Show InChI InChI=1S/C20H26F3N5O3/c1-26-12-19(4-5-19)10-14(17(29)25-31)16(26)18(30)28-8-6-27(7-9-28)15-3-2-13(11-24-15)20(21,22)23/h2-3,11,14,16,31H,4-10,12H2,1H3,(H,25,29)/t14-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human MMP7 expressed in Escherichia coli using MOCAc-Pro-Leu-Gly-Leu-A2pr(Dnp)-Ala-Arg-NH2 as substrate by fluorescence spe... |
ACS Med Chem Lett 9: 708-713 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00163
BindingDB Entry DOI: 10.7270/Q25M688J |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50284751

(CHEMBL30804 | [(S)-4-Methyl-2-((S)-9-oxo-1,8-diaza...)Show SMILES CC(C)C[C@H](CP(O)(=O)CSc1ccc2ccccc2n1)C(=O)N[C@H]1Cc2cn(CCCCCCNC1=O)c1ccccc21 Show InChI InChI=1S/C34H43N4O4PS/c1-24(2)19-27(22-43(41,42)23-44-32-16-15-25-11-5-7-13-29(25)36-32)33(39)37-30-20-26-21-38(31-14-8-6-12-28(26)31)18-10-4-3-9-17-35-34(30)40/h5-8,11-16,21,24,27,30H,3-4,9-10,17-20,22-23H2,1-2H3,(H,35,40)(H,37,39)(H,41,42)/t27-,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-7 |
Bioorg Med Chem Lett 5: 1415-1420 (1995)
Article DOI: 10.1016/0960-894X(95)00233-J
BindingDB Entry DOI: 10.7270/Q2Z89CBH |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50028945
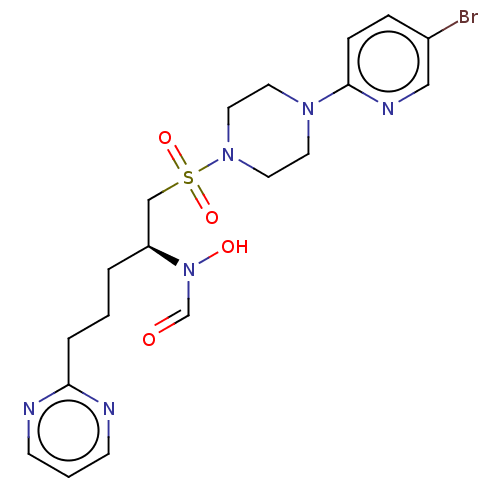
(CHEMBL1738911)Show SMILES ON(C=O)[C@@H](CCCc1ncccn1)CS(=O)(=O)N1CCN(CC1)c1ccc(Br)cn1 |r| Show InChI InChI=1S/C19H25BrN6O4S/c20-16-5-6-19(23-13-16)24-9-11-25(12-10-24)31(29,30)14-17(26(28)15-27)3-1-4-18-21-7-2-8-22-18/h2,5-8,13,15,17,28H,1,3-4,9-12,14H2/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad CEU San Pablo
Curated by ChEMBL
| Assay Description
Inhibition of MMP7 (unknown origin) |
J Med Chem 57: 10205-19 (2014)
Article DOI: 10.1021/jm500505f
BindingDB Entry DOI: 10.7270/Q2NK3GMC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Matrilysin
(Homo sapiens (Human)) | BDBM50433880

(CHEMBL2380387)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](CC(C)C)S[C@H]1CN(C[C@H]1S)C(C)=O |r| Show InChI InChI=1S/C22H33N3O3S2/c1-14(2)10-19(30-20-13-25(15(3)26)12-18(20)29)22(28)24-17(21(27)23-4)11-16-8-6-5-7-9-16/h5-9,14,17-20,29H,10-13H2,1-4H3,(H,23,27)(H,24,28)/t17-,18+,19+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida State University
Curated by ChEMBL
| Assay Description
Inhibition of MMP-7 (unknown origin) using Mca-PLGLDpa-AR-NH2 as substrate preincubated for 30 mins by fluorescence assay |
J Med Chem 56: 4357-73 (2013)
Article DOI: 10.1021/jm400529f
BindingDB Entry DOI: 10.7270/Q21Z45TX |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50284754

((S)-2-Mercaptomethyl-4-methyl-pentanoic acid ((S)-...)Show SMILES CC(C)C[C@H](CS)C(=O)N[C@H]1Cc2cn(CCCCCCNC1=O)c1ccccc21 Show InChI InChI=1S/C24H35N3O2S/c1-17(2)13-19(16-30)23(28)26-21-14-18-15-27(22-10-6-5-9-20(18)22)12-8-4-3-7-11-25-24(21)29/h5-6,9-10,15,17,19,21,30H,3-4,7-8,11-14,16H2,1-2H3,(H,25,29)(H,26,28)/t19-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-7 |
Bioorg Med Chem Lett 5: 1415-1420 (1995)
Article DOI: 10.1016/0960-894X(95)00233-J
BindingDB Entry DOI: 10.7270/Q2Z89CBH |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50078935
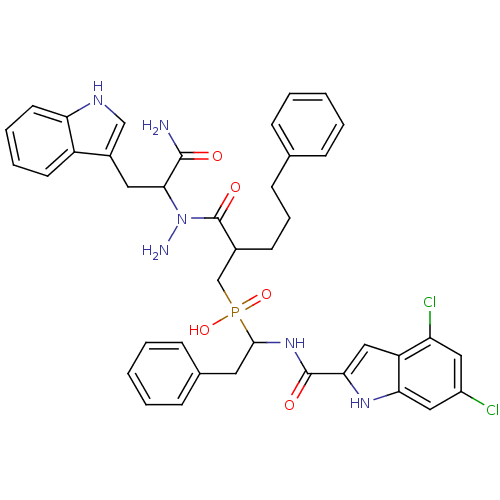
((2-{N-[1-Carbamoyl-2-(1H-indol-3-yl)-ethyl]-hydraz...)Show SMILES NN(C(Cc1c[nH]c2ccccc12)C(N)=O)C(=O)C(CCCc1ccccc1)CP(O)(=O)C(Cc1ccccc1)NC(=O)c1cc2c(Cl)cc(Cl)cc2[nH]1 Show InChI InChI=1S/C40H41Cl2N6O5P/c41-29-20-32(42)31-22-35(46-34(31)21-29)39(50)47-37(18-26-12-5-2-6-13-26)54(52,53)24-27(15-9-14-25-10-3-1-4-11-25)40(51)48(44)36(38(43)49)19-28-23-45-33-17-8-7-16-30(28)33/h1-8,10-13,16-17,20-23,27,36-37,45-46H,9,14-15,18-19,24,44H2,(H2,43,49)(H,47,50)(H,52,53) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloprotease-7 Matrilysin |
J Med Chem 42: 2610-20 (1999)
Article DOI: 10.1021/jm9900164
BindingDB Entry DOI: 10.7270/Q22B8X71 |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50104973
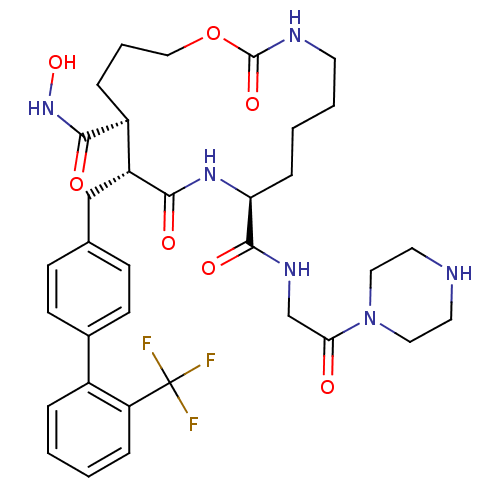
(2,10-Dioxo-11-(2'-trifluoromethyl-biphenyl-4-ylmet...)Show SMILES ONC(=O)[C@H]1CCCOC(=O)NCCCC[C@H](NC(=O)[C@@H]1Cc1ccc(cc1)-c1ccccc1C(F)(F)F)C(=O)NCC(=O)N1CCNCC1 Show InChI InChI=1S/C34H43F3N6O7/c35-34(36,37)27-8-2-1-6-24(27)23-12-10-22(11-13-23)20-26-25(31(46)42-49)7-5-19-50-33(48)39-14-4-3-9-28(41-30(26)45)32(47)40-21-29(44)43-17-15-38-16-18-43/h1-2,6,8,10-13,25-26,28,38,49H,3-5,7,9,14-21H2,(H,39,48)(H,40,47)(H,41,45)(H,42,46)/t25-,26+,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 177 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-7 |
J Med Chem 44: 3351-4 (2001)
BindingDB Entry DOI: 10.7270/Q2VD6XRT |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50393207
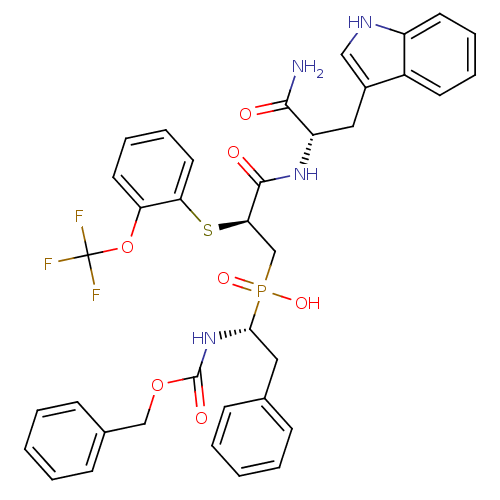
(CHEMBL2153738)Show SMILES NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](CP(O)(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1)Sc1ccccc1OC(F)(F)F |r| Show InChI InChI=1S/C37H36F3N4O7PS/c38-37(39,40)51-30-17-9-10-18-31(30)53-32(35(46)43-29(34(41)45)20-26-21-42-28-16-8-7-15-27(26)28)23-52(48,49)33(19-24-11-3-1-4-12-24)44-36(47)50-22-25-13-5-2-6-14-25/h1-18,21,29,32-33,42H,19-20,22-23H2,(H2,41,45)(H,43,46)(H,44,47)(H,48,49)/t29-,32+,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of MMP-7 |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50078949

((1-Benzyloxycarbonylamino-2-phenyl-ethyl)-{2-[1-ca...)Show SMILES CC(C)CC(CP(O)(=O)C(Cc1ccccc1)NC(=O)OCc1ccccc1)C(=O)NC(Cc1c[nH]c2ccccc12)C(N)=O Show InChI InChI=1S/C34H41N4O6P/c1-23(2)17-27(33(40)37-30(32(35)39)19-26-20-36-29-16-10-9-15-28(26)29)22-45(42,43)31(18-24-11-5-3-6-12-24)38-34(41)44-21-25-13-7-4-8-14-25/h3-16,20,23,27,30-31,36H,17-19,21-22H2,1-2H3,(H2,35,39)(H,37,40)(H,38,41)(H,42,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloprotease-7 Matrilysin |
J Med Chem 42: 2610-20 (1999)
Article DOI: 10.1021/jm9900164
BindingDB Entry DOI: 10.7270/Q22B8X71 |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50433881

(CHEMBL2380406)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](CC(C)C)S[C@H]1CNC[C@H]1S |r| Show InChI InChI=1S/C20H31N3O2S2/c1-13(2)9-17(27-18-12-22-11-16(18)26)20(25)23-15(19(24)21-3)10-14-7-5-4-6-8-14/h4-8,13,15-18,22,26H,9-12H2,1-3H3,(H,21,24)(H,23,25)/t15-,16+,17+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida State University
Curated by ChEMBL
| Assay Description
Inhibition of MMP-7 (unknown origin) using Mca-PLGLDpa-AR-NH2 as substrate preincubated for 30 mins by fluorescence assay |
J Med Chem 56: 4357-73 (2013)
Article DOI: 10.1021/jm400529f
BindingDB Entry DOI: 10.7270/Q21Z45TX |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50260828
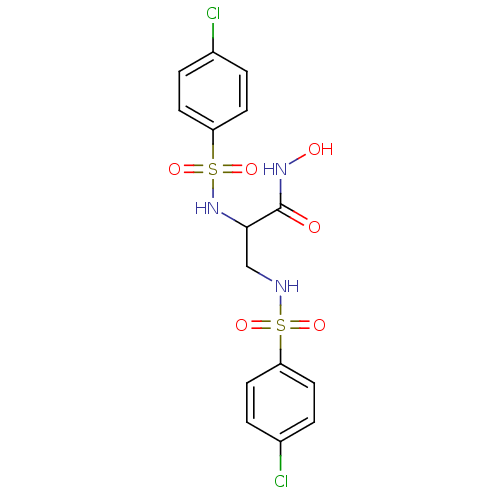
(2,3-bis(4-chlorophenylsulfonamido)-N-hydroxypropan...)Show SMILES ONC(=O)C(CNS(=O)(=O)c1ccc(Cl)cc1)NS(=O)(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C15H15Cl2N3O6S2/c16-10-1-5-12(6-2-10)27(23,24)18-9-14(15(21)19-22)20-28(25,26)13-7-3-11(17)4-8-13/h1-8,14,18,20,22H,9H2,(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 237 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP7 |
Bioorg Med Chem Lett 18: 3333-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.035
BindingDB Entry DOI: 10.7270/Q2SX6D1R |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM26526
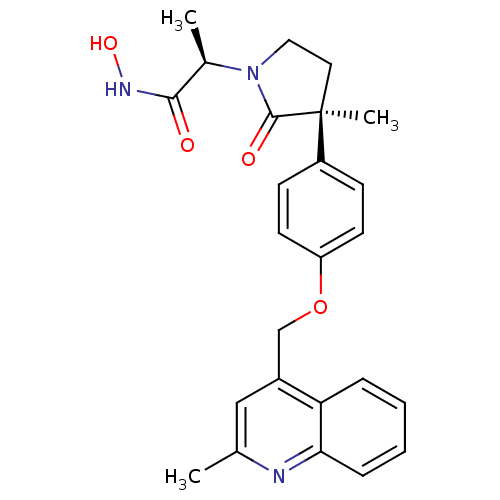
((2R)-N-hydroxy-2-[(3S)-3-methyl-3-{4-[(2-methylqui...)Show SMILES C[C@@H](N1CC[C@](C)(C1=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)C(=O)NO |r| Show InChI InChI=1S/C25H27N3O4/c1-16-14-18(21-6-4-5-7-22(21)26-16)15-32-20-10-8-19(9-11-20)25(3)12-13-28(24(25)30)17(2)23(29)27-31/h4-11,14,17,31H,12-13,15H2,1-3H3,(H,27,29)/t17-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 259 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Affinity for Matrix metalloprotease-7 (MMP-7) |
J Med Chem 45: 4954-7 (2002)
BindingDB Entry DOI: 10.7270/Q2XP7497 |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM26526

((2R)-N-hydroxy-2-[(3S)-3-methyl-3-{4-[(2-methylqui...)Show SMILES C[C@@H](N1CC[C@](C)(C1=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)C(=O)NO |r| Show InChI InChI=1S/C25H27N3O4/c1-16-14-18(21-6-4-5-7-22(21)26-16)15-32-20-10-8-19(9-11-20)25(3)12-13-28(24(25)30)17(2)23(29)27-31/h4-11,14,17,31H,12-13,15H2,1-3H3,(H,27,29)/t17-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 259 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of MMP7 |
Bioorg Med Chem 16: 8781-94 (2008)
Article DOI: 10.1016/j.bmc.2008.08.058
BindingDB Entry DOI: 10.7270/Q2JD4WM2 |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM26526

((2R)-N-hydroxy-2-[(3S)-3-methyl-3-{4-[(2-methylqui...)Show SMILES C[C@@H](N1CC[C@](C)(C1=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)C(=O)NO |r| Show InChI InChI=1S/C25H27N3O4/c1-16-14-18(21-6-4-5-7-22(21)26-16)15-32-20-10-8-19(9-11-20)25(3)12-13-28(24(25)30)17(2)23(29)27-31/h4-11,14,17,31H,12-13,15H2,1-3H3,(H,27,29)/t17-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 259 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to MMP7 |
Bioorg Med Chem Lett 17: 2769-74 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.076
BindingDB Entry DOI: 10.7270/Q2ZC82J4 |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50260884

(2,3-bis(2,4-dimethoxyphenylsulfonamido)-N-hydroxyp...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)NCC(NS(=O)(=O)c1ccc(OC)cc1OC)C(=O)NO Show InChI InChI=1S/C19H25N3O10S2/c1-29-12-5-7-17(15(9-12)31-3)33(25,26)20-11-14(19(23)21-24)22-34(27,28)18-8-6-13(30-2)10-16(18)32-4/h5-10,14,20,22,24H,11H2,1-4H3,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 289 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP7 |
Bioorg Med Chem Lett 18: 3333-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.035
BindingDB Entry DOI: 10.7270/Q2SX6D1R |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM26561
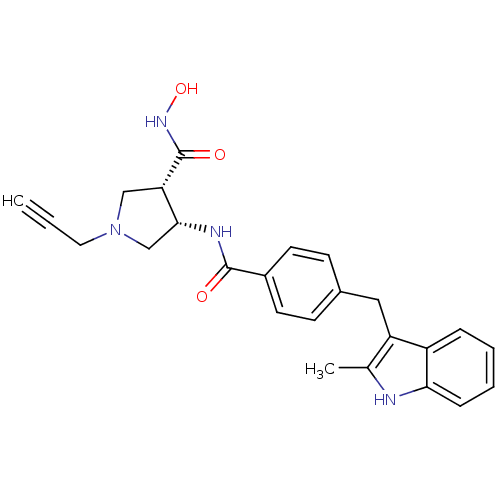
((3S,4S)-3-N-hydroxy-4-N-{4-[(2-methyl-1H-indol-3-y...)Show SMILES Cc1[nH]c2ccccc2c1Cc1ccc(cc1)C(=O)N[C@@H]1CN(CC#C)C[C@@H]1C(=O)NO |r| Show InChI InChI=1S/C25H26N4O3/c1-3-12-29-14-21(25(31)28-32)23(15-29)27-24(30)18-10-8-17(9-11-18)13-20-16(2)26-22-7-5-4-6-19(20)22/h1,4-11,21,23,26,32H,12-15H2,2H3,(H,27,30)(H,28,31)/t21-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 311 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
| Assay Description
The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... |
Bioorg Med Chem Lett 18: 1958-62 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.120
BindingDB Entry DOI: 10.7270/Q25B00SX |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50555049
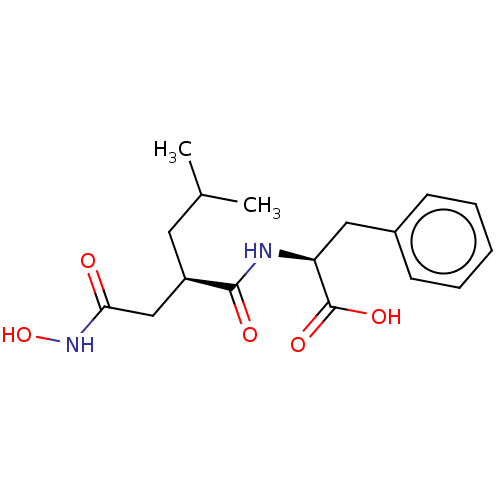
(CHEMBL4747528)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 323 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant MMP7 using Mca-Lys-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01514
BindingDB Entry DOI: 10.7270/Q2PC361P |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50332948

((R)-2-(N-((S)-2,3-dihydroxypropyl)-4-methoxyphenyl...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(C[C@H](O)CO)[C@H](CO)C(=O)NO |r| Show InChI InChI=1S/C13H20N2O8S/c1-23-10-2-4-11(5-3-10)24(21,22)15(6-9(18)7-16)12(8-17)13(19)14-20/h2-5,9,12,16-18,20H,6-8H2,1H3,(H,14,19)/t9-,12+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 344 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ProtEra s.r.l.
Curated by ChEMBL
| Assay Description
Inhibition of MMP7 by fluorimetric assay |
Eur J Med Chem 45: 5919-25 (2010)
Article DOI: 10.1016/j.ejmech.2010.09.057
BindingDB Entry DOI: 10.7270/Q29G5N2W |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50078932
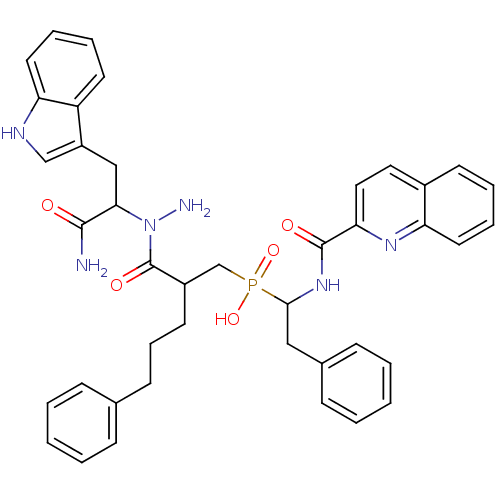
((2-{N-[1-Carbamoyl-2-(1H-indol-3-yl)-ethyl]-hydraz...)Show SMILES NN(C(Cc1c[nH]c2ccccc12)C(N)=O)C(=O)C(CCCc1ccccc1)CP(O)(=O)C(Cc1ccccc1)NC(=O)c1ccc2ccccc2n1 Show InChI InChI=1S/C41H43N6O5P/c42-39(48)37(25-32-26-44-35-21-10-8-19-33(32)35)47(43)41(50)31(18-11-16-28-12-3-1-4-13-28)27-53(51,52)38(24-29-14-5-2-6-15-29)46-40(49)36-23-22-30-17-7-9-20-34(30)45-36/h1-10,12-15,17,19-23,26,31,37-38,44H,11,16,18,24-25,27,43H2,(H2,42,48)(H,46,49)(H,51,52) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloprotease-7 Matrilysin |
J Med Chem 42: 2610-20 (1999)
Article DOI: 10.1021/jm9900164
BindingDB Entry DOI: 10.7270/Q22B8X71 |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM120212
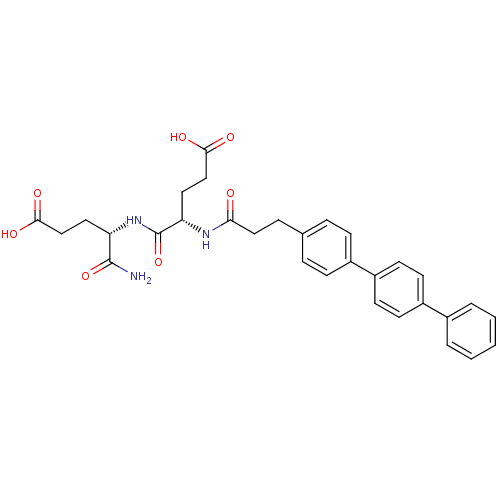
(US8691753, 61 | US8691753, 72)Show SMILES NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CCc1ccc(cc1)-c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C31H33N3O7/c32-30(40)25(15-18-28(36)37)34-31(41)26(16-19-29(38)39)33-27(35)17-8-20-6-9-22(10-7-20)24-13-11-23(12-14-24)21-4-2-1-3-5-21/h1-7,9-14,25-26H,8,15-19H2,(H2,32,40)(H,33,35)(H,34,41)(H,36,37)(H,38,39)/t25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 502 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives
US Patent
| Assay Description
The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... |
US Patent US8691753 (2014)
BindingDB Entry DOI: 10.7270/Q28P5Z5Q |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50246792

(CHEMBL508335 | N'-{3-(R)-[2-(S)-(1H-Indol-3-yl)-1-...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NNS(=O)(=O)c1ccc(cc1)-c1ccc(Br)cc1 |r| Show InChI InChI=1S/C32H36BrN5O5S/c1-20(2)16-23(31(40)36-29(32(41)34-3)17-24-19-35-28-7-5-4-6-27(24)28)18-30(39)37-38-44(42,43)26-14-10-22(11-15-26)21-8-12-25(33)13-9-21/h4-15,19-20,23,29,35,38H,16-18H2,1-3H3,(H,34,41)(H,36,40)(H,37,39)/t23-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 523 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant pro-MMP7 by spectrofluorimeter |
Bioorg Med Chem 16: 8745-59 (2008)
Article DOI: 10.1016/j.bmc.2008.07.041
BindingDB Entry DOI: 10.7270/Q2R49QM0 |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50260799

(CHEMBL497962 | N-hydroxy-2,3-bis(3-(trifluoromethy...)Show SMILES ONC(=O)C(CNS(=O)(=O)c1cccc(c1)C(F)(F)F)NS(=O)(=O)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C17H15F6N3O6S2/c18-16(19,20)10-3-1-5-12(7-10)33(29,30)24-9-14(15(27)25-28)26-34(31,32)13-6-2-4-11(8-13)17(21,22)23/h1-8,14,24,26,28H,9H2,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 602 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP7 |
Bioorg Med Chem Lett 18: 3333-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.035
BindingDB Entry DOI: 10.7270/Q2SX6D1R |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50078950
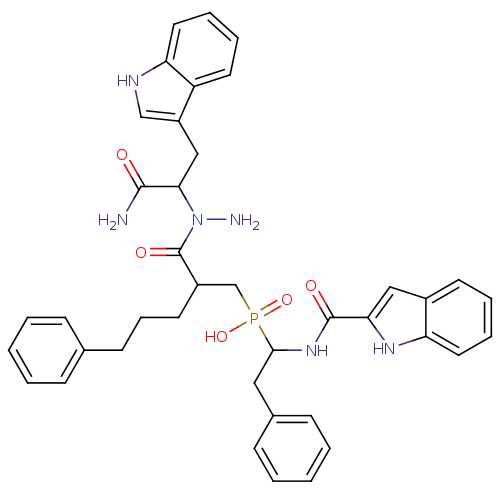
((2-{N-[1-Carbamoyl-2-(1H-indol-3-yl)-ethyl]-hydraz...)Show SMILES NN(C(Cc1c[nH]c2ccccc12)C(N)=O)C(=O)C(CCCc1ccccc1)CP(O)(=O)C(Cc1ccccc1)NC(=O)c1cc2ccccc2[nH]1 Show InChI InChI=1S/C40H43N6O5P/c41-38(47)36(24-31-25-43-34-21-10-8-19-32(31)34)46(42)40(49)30(18-11-16-27-12-3-1-4-13-27)26-52(50,51)37(22-28-14-5-2-6-15-28)45-39(48)35-23-29-17-7-9-20-33(29)44-35/h1-10,12-15,17,19-21,23,25,30,36-37,43-44H,11,16,18,22,24,26,42H2,(H2,41,47)(H,45,48)(H,50,51) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 605 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloprotease-7 Matrilysin |
J Med Chem 42: 2610-20 (1999)
Article DOI: 10.1021/jm9900164
BindingDB Entry DOI: 10.7270/Q22B8X71 |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50260826
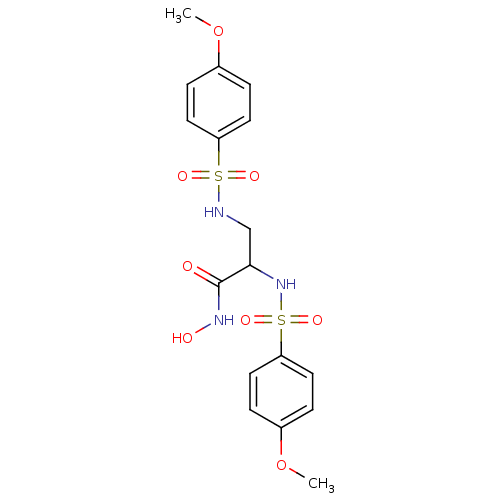
(CHEMBL497761 | N-hydroxy-2,3-bis(4-methoxyphenylsu...)Show SMILES COc1ccc(cc1)S(=O)(=O)NCC(NS(=O)(=O)c1ccc(OC)cc1)C(=O)NO Show InChI InChI=1S/C17H21N3O8S2/c1-27-12-3-7-14(8-4-12)29(23,24)18-11-16(17(21)19-22)20-30(25,26)15-9-5-13(28-2)6-10-15/h3-10,16,18,20,22H,11H2,1-2H3,(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 605 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP7 |
Bioorg Med Chem Lett 18: 3333-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.035
BindingDB Entry DOI: 10.7270/Q2SX6D1R |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50555048

(CHEMBL4793938)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(O)=O)C(=O)NCCNC(=O)c1ccc(NN)nc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 615 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant MMP7 using Mca-Lys-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01514
BindingDB Entry DOI: 10.7270/Q2PC361P |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50284757
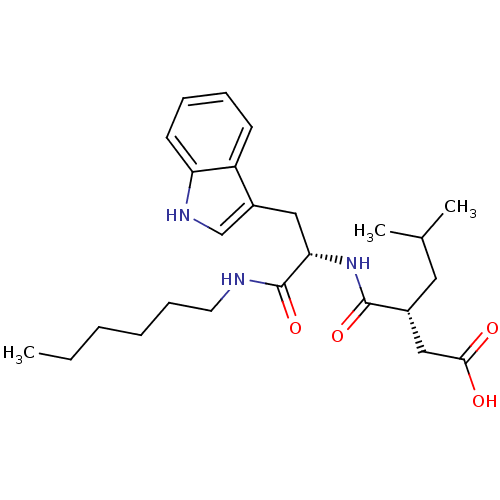
((R)-3-[(S)-1-Hexylcarbamoyl-2-(1H-indol-3-yl)-ethy...)Show SMILES CCCCCCNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(O)=O Show InChI InChI=1S/C25H37N3O4/c1-4-5-6-9-12-26-25(32)22(14-19-16-27-21-11-8-7-10-20(19)21)28-24(31)18(13-17(2)3)15-23(29)30/h7-8,10-11,16-18,22,27H,4-6,9,12-15H2,1-3H3,(H,26,32)(H,28,31)(H,29,30)/t18-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| >620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-7 |
Bioorg Med Chem Lett 5: 1415-1420 (1995)
Article DOI: 10.1016/0960-894X(95)00233-J
BindingDB Entry DOI: 10.7270/Q2Z89CBH |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50260800

(2,3-bis(4-fluorophenylsulfonamido)-N-hydroxypropan...)Show SMILES ONC(=O)C(CNS(=O)(=O)c1ccc(F)cc1)NS(=O)(=O)c1ccc(F)cc1 Show InChI InChI=1S/C15H15F2N3O6S2/c16-10-1-5-12(6-2-10)27(23,24)18-9-14(15(21)19-22)20-28(25,26)13-7-3-11(17)4-8-13/h1-8,14,18,20,22H,9H2,(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 625 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP7 |
Bioorg Med Chem Lett 18: 3333-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.035
BindingDB Entry DOI: 10.7270/Q2SX6D1R |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM92441

(RXP470, 1 | RXP470, Compound 4)Show SMILES NC(=O)[C@@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)C(Cc1cc(no1)-c1ccc(cc1)-c1cccc(Cl)c1)CP(O)(=O)c1ccc(Br)cc1 |r| Show InChI InChI=1S/C35H35BrClN4O10P/c36-24-8-10-27(11-9-24)52(49,50)19-23(34(47)40-29(13-15-32(44)45)35(48)39-28(33(38)46)12-14-31(42)43)17-26-18-30(41-51-26)21-6-4-20(5-7-21)22-2-1-3-25(37)16-22/h1-11,16,18,23,28-29H,12-15,17,19H2,(H2,38,46)(H,39,48)(H,40,47)(H,42,43)(H,44,45)(H,49,50)/t23?,28-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 626 | -35.4 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
Commissariat à l'Energie Atomique
| Assay Description
Enzyme assay using matrix metalloproteinases. |
J Biol Chem 285: 35900-9 (2010)
Article DOI: 10.1074/jbc.M110.139634
BindingDB Entry DOI: 10.7270/Q25X27HV |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50265078
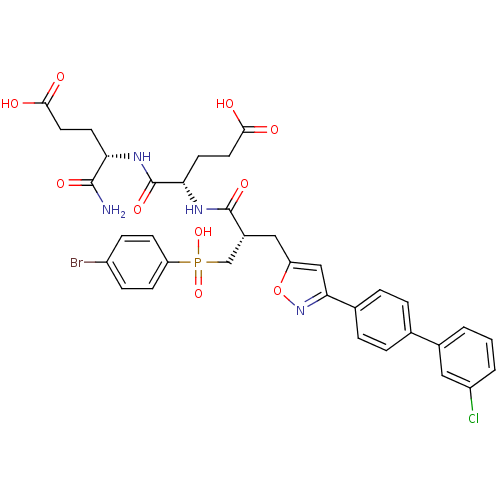
((4S)-5-amino-4-((2S)-2-((2S)-3-((4-bromophenyl)(hy...)Show SMILES NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1cc(no1)-c1ccc(cc1)-c1cccc(Cl)c1)CP(O)(=O)c1ccc(Br)cc1 |r| Show InChI InChI=1S/C35H35BrClN4O10P/c36-24-8-10-27(11-9-24)52(49,50)19-23(34(47)40-29(13-15-32(44)45)35(48)39-28(33(38)46)12-14-31(42)43)17-26-18-30(41-51-26)21-6-4-20(5-7-21)22-2-1-3-25(37)16-22/h1-11,16,18,23,28-29H,12-15,17,19H2,(H2,38,46)(H,39,48)(H,40,47)(H,42,43)(H,44,45)(H,49,50)/t23-,28+,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 626 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of MMP7 |
Bioorg Med Chem 16: 8781-94 (2008)
Article DOI: 10.1016/j.bmc.2008.08.058
BindingDB Entry DOI: 10.7270/Q2JD4WM2 |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50592891

(CHEMBL5191016)Show SMILES NC(=O)[C@@H](CCC(O)=O)NC(=O)[C@@H](CCC(O)=O)NC(=O)[C@@H](Cc1cc(no1)-c1ccc(cc1)-c1cccc(Cl)c1)CP(O)(=O)c1ccc(Br)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 626 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01855
BindingDB Entry DOI: 10.7270/Q2GB281X |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50216004
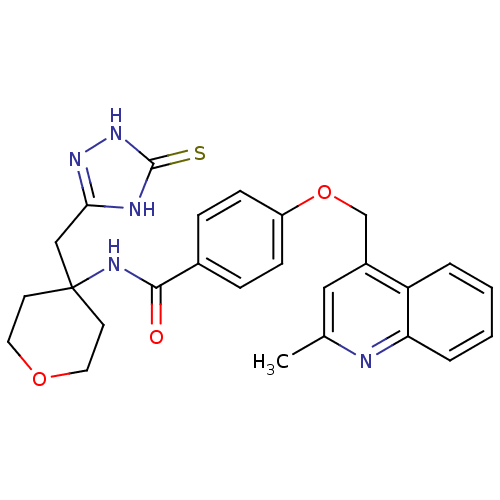
(4-((2-methylquinolin-4-yl)methoxy)-N-(4-((5-thioxo...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NC2(Cc3n[nH]c(=S)[nH]3)CCOCC2)c2ccccc2n1 Show InChI InChI=1S/C26H27N5O3S/c1-17-14-19(21-4-2-3-5-22(21)27-17)16-34-20-8-6-18(7-9-20)24(32)29-26(10-12-33-13-11-26)15-23-28-25(35)31-30-23/h2-9,14H,10-13,15-16H2,1H3,(H,29,32)(H2,28,30,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 635 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP7 |
Bioorg Med Chem Lett 17: 4678-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.100
BindingDB Entry DOI: 10.7270/Q2H41R4X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data