

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM23953 (5-(4-phenylbenzyl)oxazole-4-carboxamide, 6a | 5-{[...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pharmacopeia Drug Discovery Inc. | Assay Description IP receptor binding activity was quantified via a filter binding assay measuring displacement of [3H]-Iloprost binding to human platelet membranes. R... | Bioorg Med Chem Lett 17: 1211-5 (2007) Article DOI: 10.1016/j.bmcl.2006.12.025 BindingDB Entry DOI: 10.7270/Q2RV0M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM240747 (US9422273, 12e) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 290 | n/a | n/a | 7.4 | 37 |
ALLERGAN, INC. US Patent | Assay Description Ca2+ signaling studies were performed using a FLIPR TETRA system (Molecular Devices, Sunnyvale, Calif., USA) in the 384-format. This is a high-throug... | US Patent US9422273 (2016) BindingDB Entry DOI: 10.7270/Q2DZ076K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM23956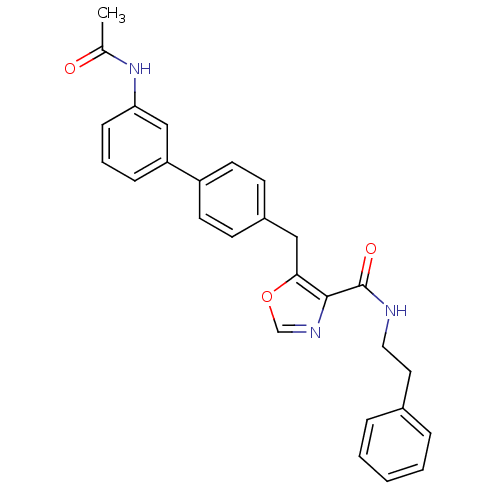 (5-(4-phenylbenzyl)oxazole-4-carboxamide, 6c | 5-{[...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 721 | n/a | 138 | n/a | n/a | 7.4 | 22 |
Pharmacopeia Drug Discovery Inc. | Assay Description IP receptor binding activity was quantified via a filter binding assay measuring displacement of [3H]-Iloprost binding to human platelet membranes. R... | Bioorg Med Chem Lett 17: 1211-5 (2007) Article DOI: 10.1016/j.bmcl.2006.12.025 BindingDB Entry DOI: 10.7270/Q2RV0M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM23957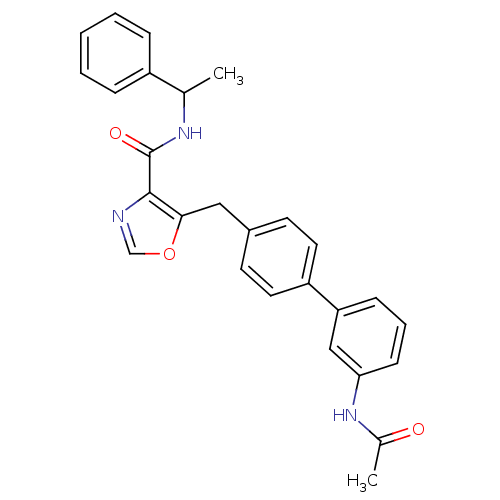 (5-(4-phenylbenzyl)oxazole-4-carboxamide, 6d | 5-{[...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.23E+3 | n/a | 278 | n/a | n/a | 7.4 | 22 |
Pharmacopeia Drug Discovery Inc. | Assay Description IP receptor binding activity was quantified via a filter binding assay measuring displacement of [3H]-Iloprost binding to human platelet membranes. R... | Bioorg Med Chem Lett 17: 1211-5 (2007) Article DOI: 10.1016/j.bmcl.2006.12.025 BindingDB Entry DOI: 10.7270/Q2RV0M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM23958 (5-(4-phenylbenzyl)oxazole-4-carboxamide, 6e | 5-{[...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.16E+3 | n/a | 2.13E+3 | n/a | n/a | 7.4 | 22 |
Pharmacopeia Drug Discovery Inc. | Assay Description IP receptor binding activity was quantified via a filter binding assay measuring displacement of [3H]-Iloprost binding to human platelet membranes. R... | Bioorg Med Chem Lett 17: 1211-5 (2007) Article DOI: 10.1016/j.bmcl.2006.12.025 BindingDB Entry DOI: 10.7270/Q2RV0M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM23959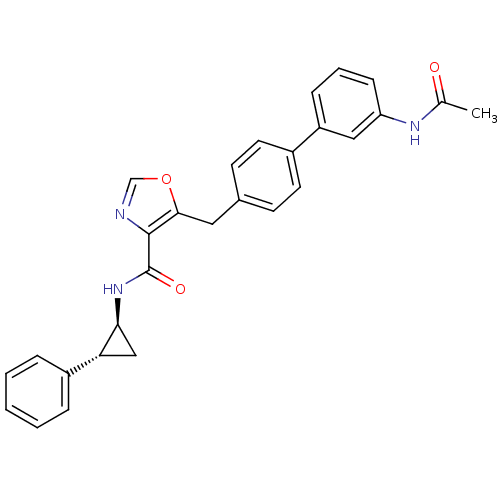 (5-(4-phenylbenzyl)oxazole-4-carboxamide, 6f | 5-{[...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 331 | n/a | 358 | n/a | n/a | 7.4 | 22 |
Pharmacopeia Drug Discovery Inc. | Assay Description IP receptor binding activity was quantified via a filter binding assay measuring displacement of [3H]-Iloprost binding to human platelet membranes. R... | Bioorg Med Chem Lett 17: 1211-5 (2007) Article DOI: 10.1016/j.bmcl.2006.12.025 BindingDB Entry DOI: 10.7270/Q2RV0M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM23960 (5-(4-phenylbenzyl)oxazole-4-carboxamide, 6g | 5-{[...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 53 | n/a | 51 | n/a | n/a | 7.4 | 22 |
Pharmacopeia Drug Discovery Inc. | Assay Description IP receptor binding activity was quantified via a filter binding assay measuring displacement of [3H]-Iloprost binding to human platelet membranes. R... | Bioorg Med Chem Lett 17: 1211-5 (2007) Article DOI: 10.1016/j.bmcl.2006.12.025 BindingDB Entry DOI: 10.7270/Q2RV0M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM23961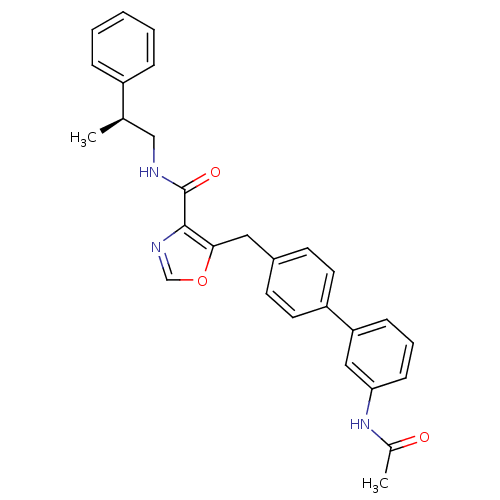 (5-(4-phenylbenzyl)oxazole-4-carboxamide, 6h | 5-{[...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 916 | n/a | 296 | n/a | n/a | 7.4 | 22 |
Pharmacopeia Drug Discovery Inc. | Assay Description IP receptor binding activity was quantified via a filter binding assay measuring displacement of [3H]-Iloprost binding to human platelet membranes. R... | Bioorg Med Chem Lett 17: 1211-5 (2007) Article DOI: 10.1016/j.bmcl.2006.12.025 BindingDB Entry DOI: 10.7270/Q2RV0M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM23962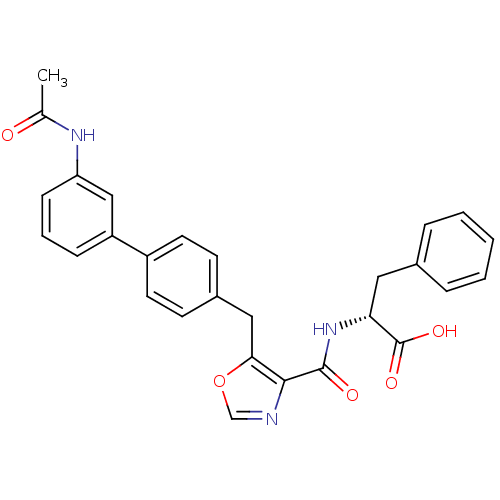 ((2R)-2-[(5-{[4-(3-acetamidophenyl)phenyl]methyl}-1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20E+3 | n/a | 831 | n/a | n/a | 7.4 | 22 |
Pharmacopeia Drug Discovery Inc. | Assay Description IP receptor binding activity was quantified via a filter binding assay measuring displacement of [3H]-Iloprost binding to human platelet membranes. R... | Bioorg Med Chem Lett 17: 1211-5 (2007) Article DOI: 10.1016/j.bmcl.2006.12.025 BindingDB Entry DOI: 10.7270/Q2RV0M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM23963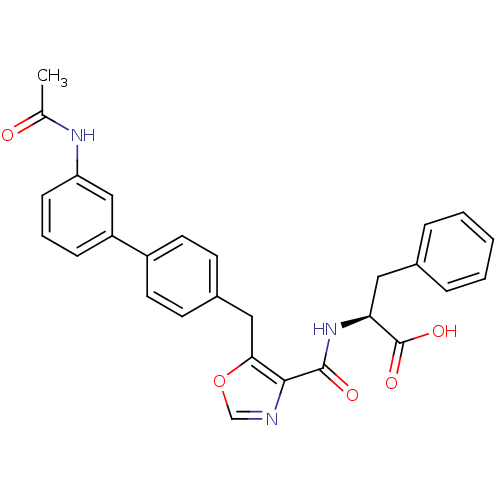 ((2S)-2-[(5-{[4-(3-acetamidophenyl)phenyl]methyl}-1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 476 | n/a | 16 | n/a | n/a | 7.4 | 22 |
Pharmacopeia Drug Discovery Inc. | Assay Description IP receptor binding activity was quantified via a filter binding assay measuring displacement of [3H]-Iloprost binding to human platelet membranes. R... | Bioorg Med Chem Lett 17: 1211-5 (2007) Article DOI: 10.1016/j.bmcl.2006.12.025 BindingDB Entry DOI: 10.7270/Q2RV0M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM23964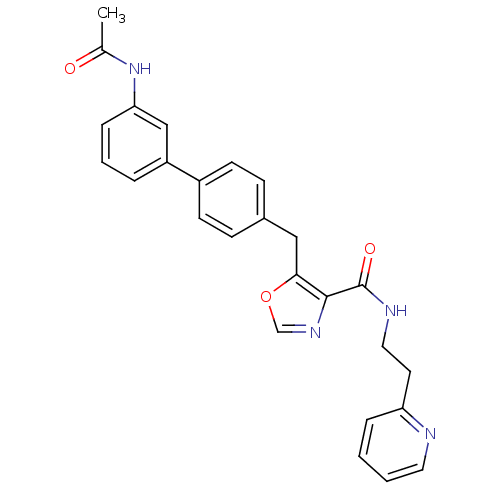 (5-(4-phenylbenzyl)oxazole-4-carboxamide, 6k | 5-{[...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.60E+3 | n/a | 1.01E+3 | n/a | n/a | 7.4 | 22 |
Pharmacopeia Drug Discovery Inc. | Assay Description IP receptor binding activity was quantified via a filter binding assay measuring displacement of [3H]-Iloprost binding to human platelet membranes. R... | Bioorg Med Chem Lett 17: 1211-5 (2007) Article DOI: 10.1016/j.bmcl.2006.12.025 BindingDB Entry DOI: 10.7270/Q2RV0M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM23965 (5-(4-phenylbenzyl)oxazole-4-carboxamide, 6l | 5-{[...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.95E+3 | n/a | 741 | n/a | n/a | 7.4 | 22 |
Pharmacopeia Drug Discovery Inc. | Assay Description IP receptor binding activity was quantified via a filter binding assay measuring displacement of [3H]-Iloprost binding to human platelet membranes. R... | Bioorg Med Chem Lett 17: 1211-5 (2007) Article DOI: 10.1016/j.bmcl.2006.12.025 BindingDB Entry DOI: 10.7270/Q2RV0M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM23966 (5-(4-phenylbenzyl)oxazole-4-carboxamide, 6m | 5-{[...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.71E+3 | n/a | 476 | n/a | n/a | 7.4 | 22 |
Pharmacopeia Drug Discovery Inc. | Assay Description IP receptor binding activity was quantified via a filter binding assay measuring displacement of [3H]-Iloprost binding to human platelet membranes. R... | Bioorg Med Chem Lett 17: 1211-5 (2007) Article DOI: 10.1016/j.bmcl.2006.12.025 BindingDB Entry DOI: 10.7270/Q2RV0M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM23967 (5-(4-phenylbenzyl)oxazole-4-carboxamide, 6n | 5-{[...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.48E+3 | n/a | 828 | n/a | n/a | 7.4 | 22 |
Pharmacopeia Drug Discovery Inc. | Assay Description IP receptor binding activity was quantified via a filter binding assay measuring displacement of [3H]-Iloprost binding to human platelet membranes. R... | Bioorg Med Chem Lett 17: 1211-5 (2007) Article DOI: 10.1016/j.bmcl.2006.12.025 BindingDB Entry DOI: 10.7270/Q2RV0M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM23968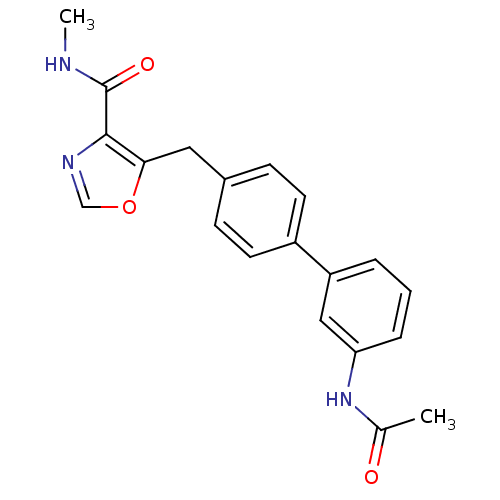 (5-(4-phenylbenzyl)oxazole-4-carboxamide, 6o | 5-{[...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.22E+4 | n/a | 3.31E+3 | n/a | n/a | 7.4 | 22 |
Pharmacopeia Drug Discovery Inc. | Assay Description IP receptor binding activity was quantified via a filter binding assay measuring displacement of [3H]-Iloprost binding to human platelet membranes. R... | Bioorg Med Chem Lett 17: 1211-5 (2007) Article DOI: 10.1016/j.bmcl.2006.12.025 BindingDB Entry DOI: 10.7270/Q2RV0M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM23969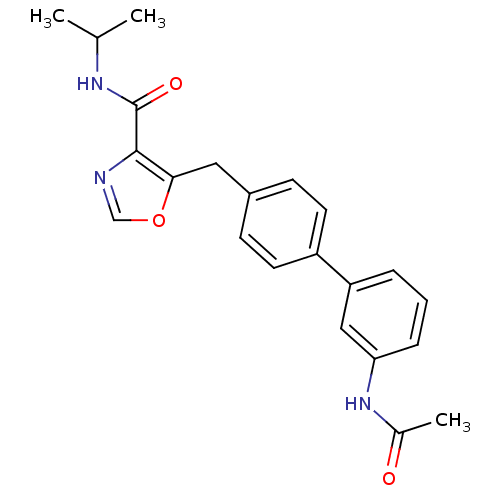 (5-(4-phenylbenzyl)oxazole-4-carboxamide, 6p | 5-{[...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.24E+4 | n/a | 3.58E+3 | n/a | n/a | 7.4 | 22 |
Pharmacopeia Drug Discovery Inc. | Assay Description IP receptor binding activity was quantified via a filter binding assay measuring displacement of [3H]-Iloprost binding to human platelet membranes. R... | Bioorg Med Chem Lett 17: 1211-5 (2007) Article DOI: 10.1016/j.bmcl.2006.12.025 BindingDB Entry DOI: 10.7270/Q2RV0M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM23970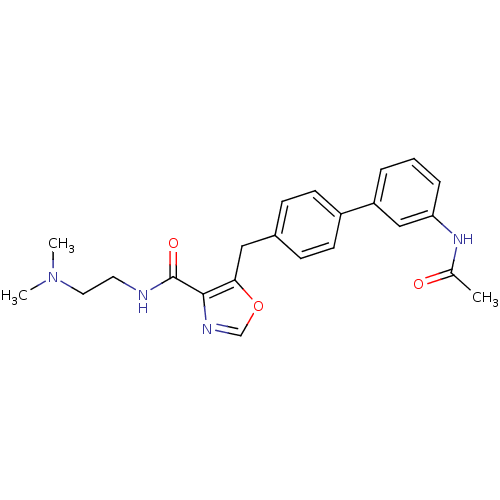 (5-(4-phenylbenzyl)oxazole-4-carboxamide, 6q | N-[2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.41E+4 | n/a | 3.31E+3 | n/a | n/a | 7.4 | 22 |
Pharmacopeia Drug Discovery Inc. | Assay Description IP receptor binding activity was quantified via a filter binding assay measuring displacement of [3H]-Iloprost binding to human platelet membranes. R... | Bioorg Med Chem Lett 17: 1211-5 (2007) Article DOI: 10.1016/j.bmcl.2006.12.025 BindingDB Entry DOI: 10.7270/Q2RV0M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM62356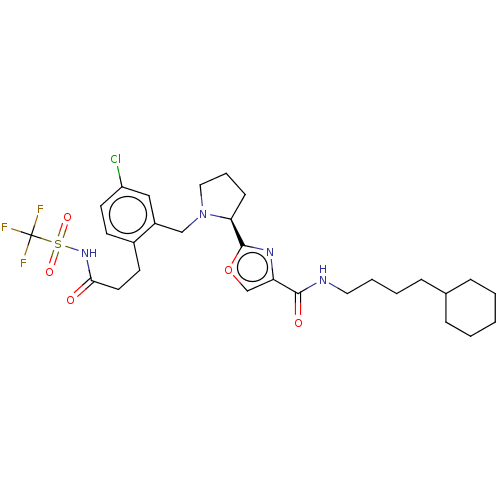 (US9422273, 12b) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 860 | n/a | n/a | 7.4 | 37 |
ALLERGAN, INC. US Patent | Assay Description Ca2+ signaling studies were performed using a FLIPR TETRA system (Molecular Devices, Sunnyvale, Calif., USA) in the 384-format. This is a high-throug... | US Patent US9422273 (2016) BindingDB Entry DOI: 10.7270/Q2DZ076K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM240744 (US9422273, 12a) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 550 | n/a | n/a | 7.4 | 37 |
ALLERGAN, INC. US Patent | Assay Description Ca2+ signaling studies were performed using a FLIPR TETRA system (Molecular Devices, Sunnyvale, Calif., USA) in the 384-format. This is a high-throug... | US Patent US9422273 (2016) BindingDB Entry DOI: 10.7270/Q2DZ076K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM240745 (US9422273, 12c) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 9.50E+3 | n/a | n/a | 7.4 | 37 |
ALLERGAN, INC. US Patent | Assay Description Ca2+ signaling studies were performed using a FLIPR TETRA system (Molecular Devices, Sunnyvale, Calif., USA) in the 384-format. This is a high-throug... | US Patent US9422273 (2016) BindingDB Entry DOI: 10.7270/Q2DZ076K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM240746 (US9422273, 12d) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | 7.4 | 37 |
ALLERGAN, INC. US Patent | Assay Description Ca2+ signaling studies were performed using a FLIPR TETRA system (Molecular Devices, Sunnyvale, Calif., USA) in the 384-format. This is a high-throug... | US Patent US9422273 (2016) BindingDB Entry DOI: 10.7270/Q2DZ076K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM23955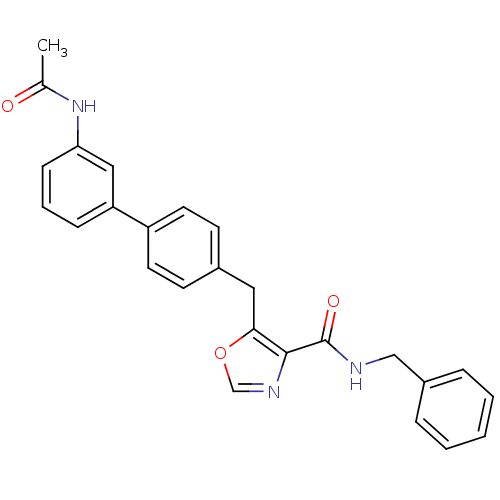 (5-(4-phenylbenzyl)oxazole-4-carboxamide, 6b | N-be...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 561 | n/a | 66 | n/a | n/a | 7.4 | 22 |
Pharmacopeia Drug Discovery Inc. | Assay Description IP receptor binding activity was quantified via a filter binding assay measuring displacement of [3H]-Iloprost binding to human platelet membranes. R... | Bioorg Med Chem Lett 17: 1211-5 (2007) Article DOI: 10.1016/j.bmcl.2006.12.025 BindingDB Entry DOI: 10.7270/Q2RV0M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50101823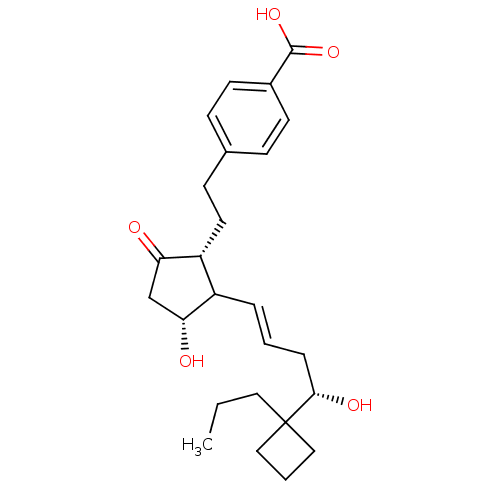 (4-(2-{(1R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4-(1-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human Prostanoid IP receptor in CHO cells. | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50101832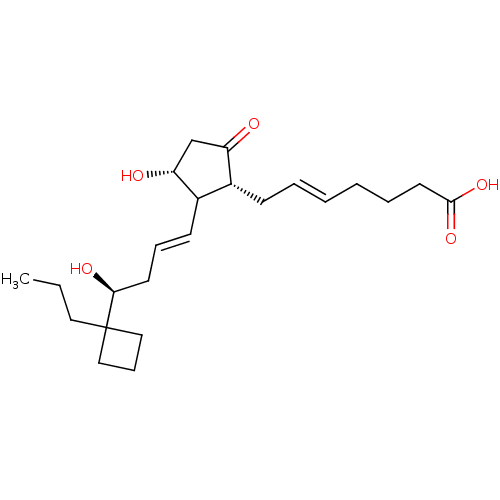 ((E)-7-{(1R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4-(1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human Prostanoid IP receptor in CHO cells. | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50101825 ((Z)-7-{(1R,2R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human Prostanoid IP receptor in CHO cells. | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50235385 (APD-811 | Ralinepag) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]-iloprost from recombinant human IP receptor expressed in CHO-K1 cell membranes incubated for 1 hr by top count scintillation cou... | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM23954 (5-[(2E,3aS,4R,5R,6aS)-5-hydroxy-4-[(1E,3S)-3-hydro...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]-iloprost from recombinant human IP receptor expressed in CHO-K1 cell membranes incubated for 1 hr by top count scintillation cou... | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50101848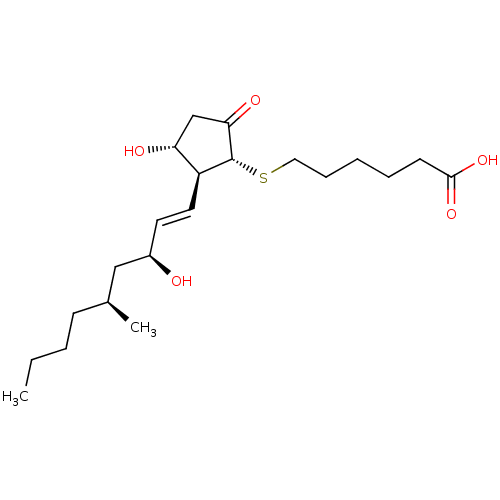 (6-[(1R,2S,3R)-3-Hydroxy-2-((E)-(3S,5S)-3-hydroxy-5...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Evaluated for its competitive binding affinity towards human Prostanoid IP receptor in CHO cells | Bioorg Med Chem Lett 11: 2029-31 (2001) BindingDB Entry DOI: 10.7270/Q2833R9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM23954 (5-[(2E,3aS,4R,5R,6aS)-5-hydroxy-4-[(1E,3S)-3-hydro...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to human PGI2 receptor by radioligand displacement assay | Eur J Med Chem 63: 85-94 (2013) Article DOI: 10.1016/j.ejmech.2013.01.044 BindingDB Entry DOI: 10.7270/Q2JH3NKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50168287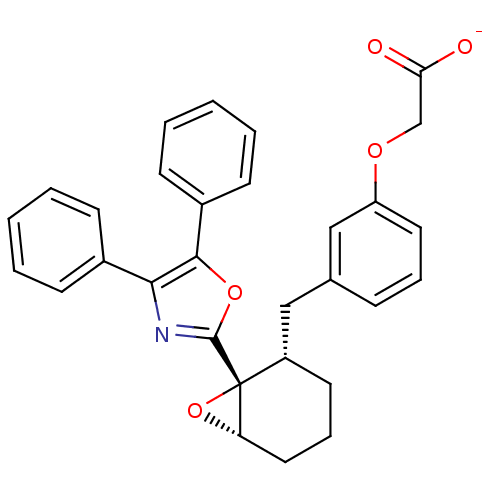 (CHEMBL363800 | Sodium; {3-[(1R,2S,6S)-1-(4,5-diphe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-Iloprost binding to human Prostanoid IP receptor | Bioorg Med Chem Lett 15: 3284-7 (2005) Article DOI: 10.1016/j.bmcl.2005.04.076 BindingDB Entry DOI: 10.7270/Q2C53KCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM23954 (5-[(2E,3aS,4R,5R,6aS)-5-hydroxy-4-[(1E,3S)-3-hydro...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Ability to inhibit binding of [3H]iloprost to cloned human prostaglandin I2 receptor | Bioorg Med Chem Lett 15: 3279-83 (2005) Article DOI: 10.1016/j.bmcl.2005.04.042 BindingDB Entry DOI: 10.7270/Q2J103X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM23954 (5-[(2E,3aS,4R,5R,6aS)-5-hydroxy-4-[(1E,3S)-3-hydro...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description In vitro Prostacyclin (PGI-2) receptor binding assay was determined based on displacement of [3H]-Iloprost radioligand from cloned human IP receptor | Bioorg Med Chem Lett 13: 4277-9 (2003) BindingDB Entry DOI: 10.7270/Q22R3R3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50235370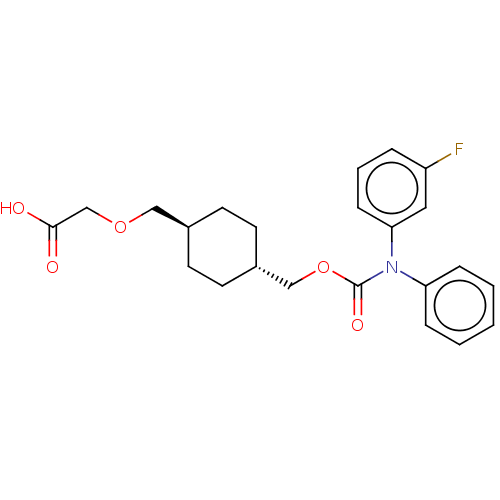 (CHEMBL3933704) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]-iloprost from recombinant human IP receptor expressed in CHO-K1 cell membranes incubated for 1 hr by top count scintillation cou... | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50167887 ((R)-2-(6-((diphenylcarbamoyloxy)methyl)-6-hydroxy-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]iloprost from human Prostanoid IP receptor | Bioorg Med Chem Lett 15: 3091-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.047 BindingDB Entry DOI: 10.7270/Q2SF2WXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM23954 (5-[(2E,3aS,4R,5R,6aS)-5-hydroxy-4-[(1E,3S)-3-hydro...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by PDSP Ki Database | Biochim Biophys Acta 1483: 285-93 (2000) Article DOI: 10.1016/s1388-1981(99)00164-x BindingDB Entry DOI: 10.7270/Q2J964XQ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50167890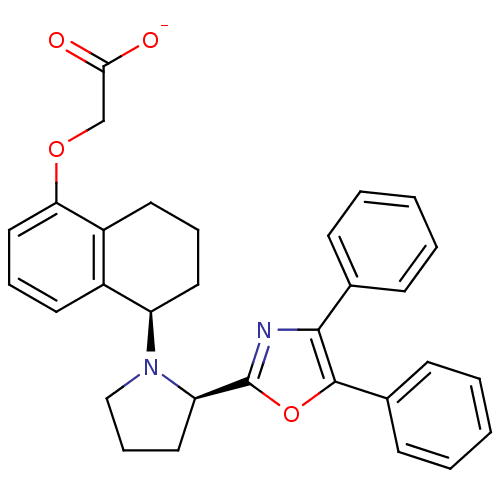 (CHEMBL190497 | FR-193262 | Sodium; {(R)-5-[(R)-2-(...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Ability to inhibit binding of [3H]iloprost to cloned human prostaglandin I2 receptor | Bioorg Med Chem Lett 15: 3279-83 (2005) Article DOI: 10.1016/j.bmcl.2005.04.042 BindingDB Entry DOI: 10.7270/Q2J103X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50167890 (CHEMBL190497 | FR-193262 | Sodium; {(R)-5-[(R)-2-(...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]iloprost from human Prostanoid IP receptor | Bioorg Med Chem Lett 15: 3091-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.047 BindingDB Entry DOI: 10.7270/Q2SF2WXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50109546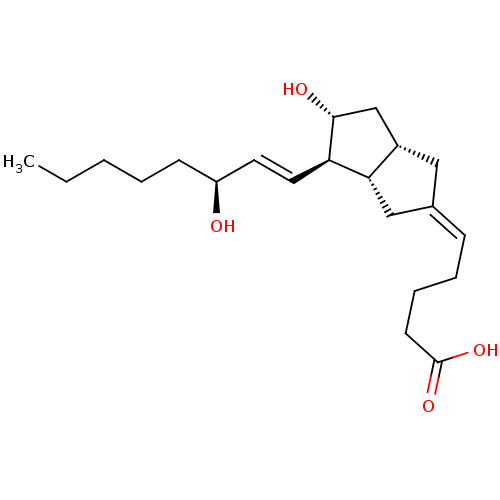 (5-[(3aS,4R,5R,6aS)-5-Hydroxy-4-((S)-3-hydroxy-oct-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 16.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Royal Postgraduate Medical School Curated by PDSP Ki Database | Br J Pharmacol 72: 435-41 (1981) Article DOI: 10.1111/j.1476-5381.1981.tb10994.x BindingDB Entry DOI: 10.7270/Q2HM56XZ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM85179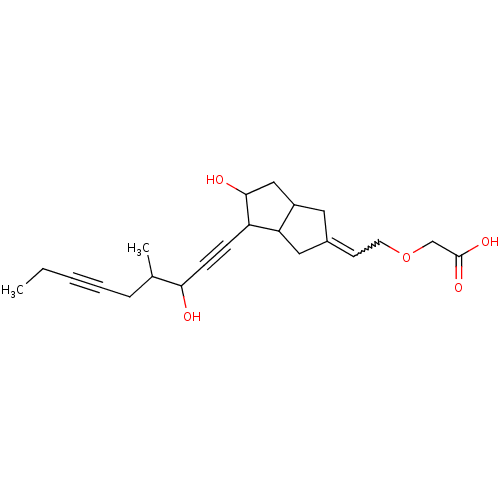 (CAS_94079-80-8 | CICAPROST | NSC_72023) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by PDSP Ki Database | Biochim Biophys Acta 1483: 285-93 (2000) Article DOI: 10.1016/s1388-1981(99)00164-x BindingDB Entry DOI: 10.7270/Q2J964XQ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50223834 (CHEMBL9910) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity towards prostacyclin receptor on rat NG-108-15 neuroblastoma cells, using [3H]iloprost as a radioligand | Bioorg Med Chem Lett 14: 1053-6 (2004) BindingDB Entry DOI: 10.7270/Q2M90BVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50223868 (CHEMBL9604) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity towards prostacyclin receptor on rat NG-108-15 neuroblastoma cells, using [3H]iloprost as a radioligand | Bioorg Med Chem Lett 14: 1053-6 (2004) BindingDB Entry DOI: 10.7270/Q2M90BVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50152515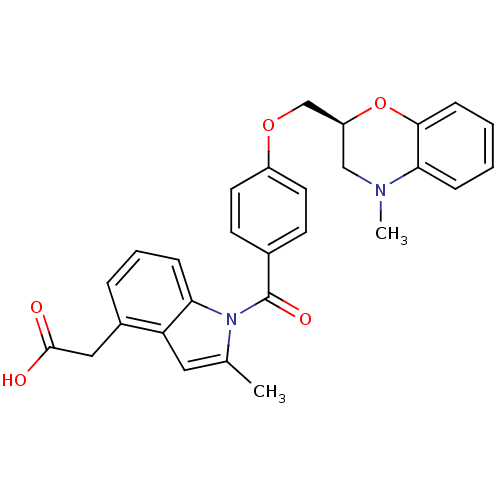 (CHEMBL364841 | {2-Methyl-1-[4-(4-methyl-3,4-dihydr...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]Iloprost from human prostanoid IP receptor expressed in CHO cells after 30 mins by liquid scintillation counting | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50152515 (CHEMBL364841 | {2-Methyl-1-[4-(4-methyl-3,4-dihydr...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]Iloprost from human prostanoid IP receptor expressed in CHO cells after 60 mins by liquid scintillation counting | Bioorg Med Chem 19: 5361-71 (2011) Article DOI: 10.1016/j.bmc.2011.08.007 BindingDB Entry DOI: 10.7270/Q2X067DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50152515 (CHEMBL364841 | {2-Methyl-1-[4-(4-methyl-3,4-dihydr...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-Iloprost from human IP receptor expressed in CHO cells after 30 mins by liquid scintillation counting | Bioorg Med Chem 19: 4574-88 (2011) Article DOI: 10.1016/j.bmc.2011.06.014 BindingDB Entry DOI: 10.7270/Q2FB5398 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50223861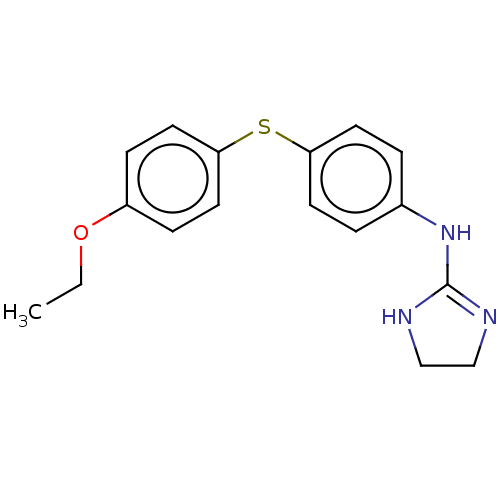 (CHEMBL9552) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity towards prostacyclin receptor on rat NG-108-15 neuroblastoma cells, using [3H]iloprost as a radioligand | Bioorg Med Chem Lett 14: 1053-6 (2004) BindingDB Entry DOI: 10.7270/Q2M90BVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50168291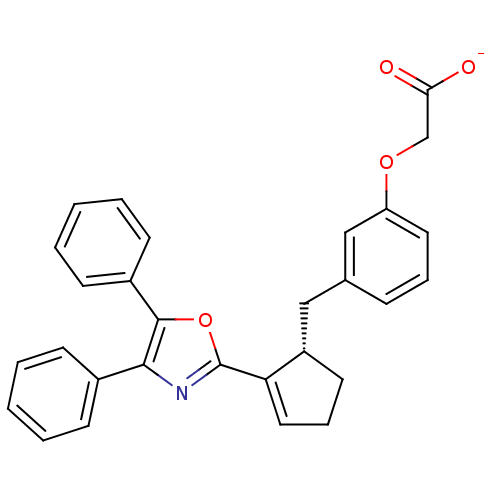 (CHEMBL363350 | Sodium; {3-[(S)-2-(4,5-diphenyl-oxa...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-Iloprost binding to human Prostanoid IP receptor | Bioorg Med Chem Lett 15: 3284-7 (2005) Article DOI: 10.1016/j.bmcl.2005.04.076 BindingDB Entry DOI: 10.7270/Q2C53KCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50350368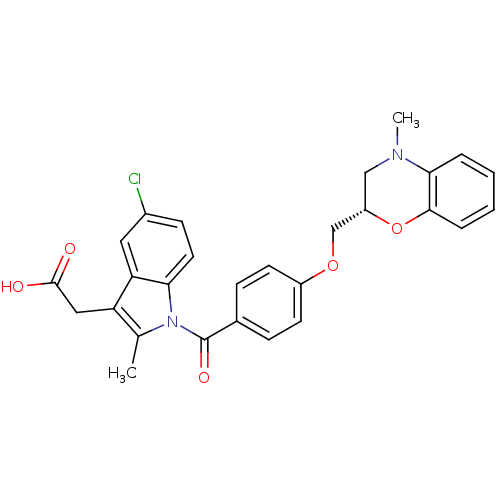 (CHEMBL1813117) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-Iloprost from human IP receptor expressed in CHO cells after 30 mins by liquid scintillation counting | Bioorg Med Chem 19: 4574-88 (2011) Article DOI: 10.1016/j.bmc.2011.06.014 BindingDB Entry DOI: 10.7270/Q2FB5398 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50223848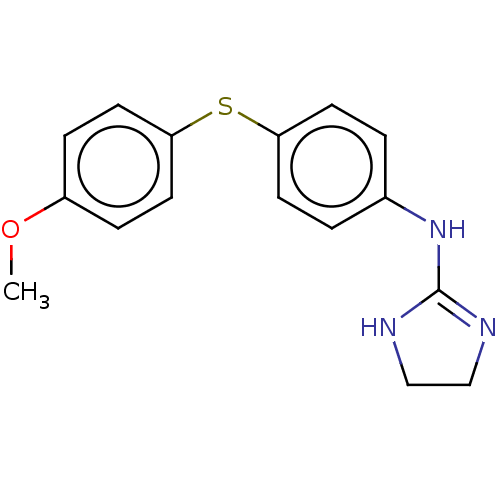 (CHEMBL10241) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity towards prostacyclin receptor on rat NG-108-15 neuroblastoma cells, using [3H]iloprost as a radioligand | Bioorg Med Chem Lett 14: 1053-6 (2004) BindingDB Entry DOI: 10.7270/Q2M90BVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50223860 (CHEMBL9846) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity towards prostacyclin receptor on rat NG-108-15 neuroblastoma cells, using [3H]iloprost as a radioligand | Bioorg Med Chem Lett 14: 1053-6 (2004) BindingDB Entry DOI: 10.7270/Q2M90BVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50223848 (CHEMBL10241) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity towards prostacyclin receptor on rat NG-108-15 neuroblastoma cells, using [3H]iloprost as a radioligand | Bioorg Med Chem Lett 14: 1053-6 (2004) BindingDB Entry DOI: 10.7270/Q2M90BVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 658 total ) | Next | Last >> |