 Found 3635 hits of Enzyme Inhibition Constant Data
Found 3635 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50344331

(CHEMBL270587 | rac-2-(3-{[(biphenyl-4-carbonyl)-am...)Show SMILES CCC(Cc1ccc(OC)c(CNC(=O)c2ccc(cc2)-c2ccccc2)c1)C(O)=O |w:2.2| Show InChI InChI=1S/C26H27NO4/c1-3-19(26(29)30)15-18-9-14-24(31-2)23(16-18)17-27-25(28)22-12-10-21(11-13-22)20-7-5-4-6-8-20/h4-14,16,19H,3,15,17H2,1-2H3,(H,27,28)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a |
Okayama University
Curated by ChEMBL
| Assay Description
Agonist activity at Gal4-fussed PPARalpha expressed in human HEK293 cells by reporter gene assay |
Bioorg Med Chem 19: 3183-91 (2011)
Article DOI: 10.1016/j.bmc.2011.03.064
BindingDB Entry DOI: 10.7270/Q2S75GNC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50179836
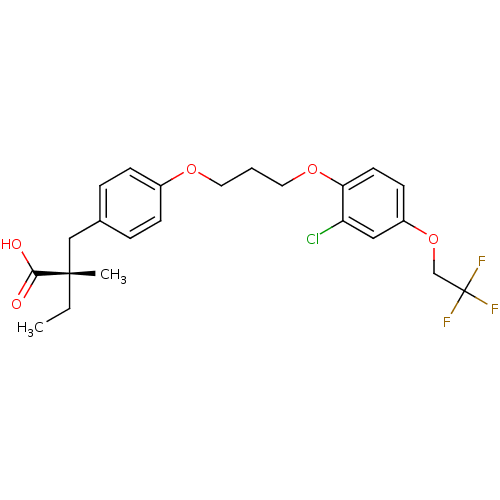
((S)-2-(4-(3-(2-chloro-4-(2,2,2-trifluoroethoxy)phe...)Show SMILES CC[C@@](C)(Cc1ccc(OCCCOc2ccc(OCC(F)(F)F)cc2Cl)cc1)C(O)=O Show InChI InChI=1S/C23H26ClF3O5/c1-3-22(2,21(28)29)14-16-5-7-17(8-6-16)30-11-4-12-31-20-10-9-18(13-19(20)24)32-15-23(25,26)27/h5-10,13H,3-4,11-12,14-15H2,1-2H3,(H,28,29)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Transactivation by human PPAR alpha in COS1 cells |
Bioorg Med Chem Lett 16: 1673-8 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.022
BindingDB Entry DOI: 10.7270/Q2930SRT |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50234375

((S)-2-ethoxy-3-(4-(2-(6-((methoxyimino)(phenyl)met...)Show SMILES CCO[C@@H](Cc1ccc(OCCN2CCC(C)(C)c3cc(ccc23)C(=N/OC)\c2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C32H38N2O5/c1-5-38-29(31(35)36)21-23-11-14-26(15-12-23)39-20-19-34-18-17-32(2,3)27-22-25(13-16-28(27)34)30(33-37-4)24-9-7-6-8-10-24/h6-16,22,29H,5,17-21H2,1-4H3,(H,35,36)/b33-30-/t29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a |
SPOT-EA3857
Curated by ChEMBL
| Assay Description
Agonist activity at human PPARalpha at 100 uM by GAL4 transactivation assay relative to WY 14,643 |
Bioorg Med Chem Lett 18: 1617-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.067
BindingDB Entry DOI: 10.7270/Q2222TGW |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50214203
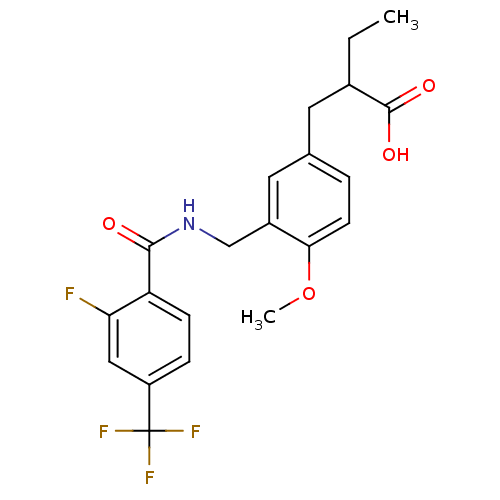
((+/-)-2-(3-((2-fluoro-4-(trifluoromethyl)benzamido...)Show SMILES CCC(Cc1ccc(OC)c(CNC(=O)c2ccc(cc2F)C(F)(F)F)c1)C(O)=O Show InChI InChI=1S/C21H21F4NO4/c1-3-13(20(28)29)8-12-4-7-18(30-2)14(9-12)11-26-19(27)16-6-5-15(10-17(16)22)21(23,24)25/h4-7,9-10,13H,3,8,11H2,1-2H3,(H,26,27)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Agonist activity at PPARalpha (unknown origin) transfected in HEK293 cells by GAL4 transactivation assay |
Bioorg Med Chem Lett 18: 4525-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.046
BindingDB Entry DOI: 10.7270/Q24X57KK |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50333774
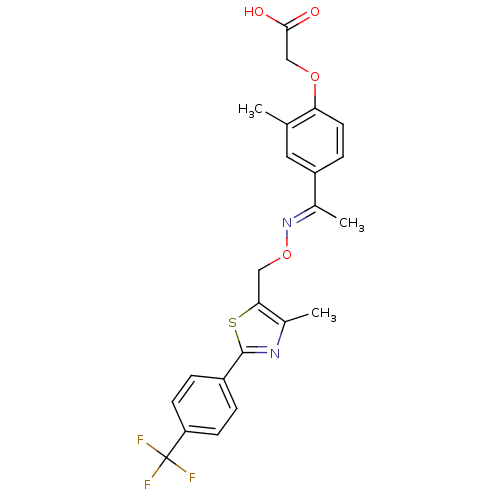
((E)-(2-Methyl-4-{1-[4-methyl-2-(4-trifluoromethylp...)Show SMILES C\C(=N/OCc1sc(nc1C)-c1ccc(cc1)C(F)(F)F)c1ccc(OCC(O)=O)c(C)c1 Show InChI InChI=1S/C23H21F3N2O4S/c1-13-10-17(6-9-19(13)31-12-21(29)30)14(2)28-32-11-20-15(3)27-22(33-20)16-4-7-18(8-5-16)23(24,25)26/h4-10H,11-12H2,1-3H3,(H,29,30)/b28-14+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Agonist activity at human PPARalpha expressed in HepG2 cells cotransfected with PPRE3-TK-luc assessed as beta-galactosidase activity after 20 to 22 h... |
Bioorg Med Chem 19: 771-82 (2011)
Article DOI: 10.1016/j.bmc.2010.12.023
BindingDB Entry DOI: 10.7270/Q2PC32MN |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50124386
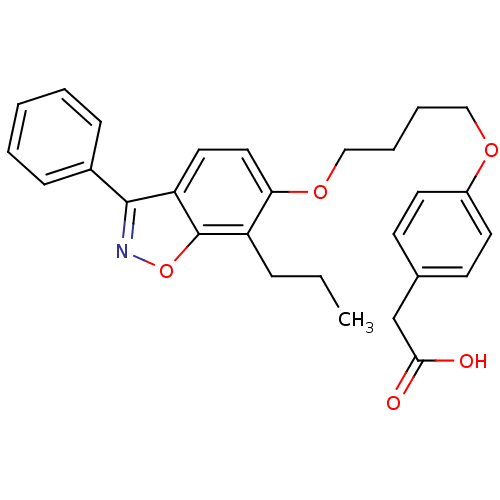
(CHEMBL367311 | {4-[4-(3-Phenyl-7-propyl-benzo[d]is...)Show SMILES CCCc1c(OCCCCOc2ccc(CC(O)=O)cc2)ccc2c(noc12)-c1ccccc1 Show InChI InChI=1S/C28H29NO5/c1-2-8-23-25(16-15-24-27(29-34-28(23)24)21-9-4-3-5-10-21)33-18-7-6-17-32-22-13-11-20(12-14-22)19-26(30)31/h3-5,9-16H,2,6-8,17-19H2,1H3,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonistic activity was determined in COS1 cells transfected with GAL 4-PPAR alpha receptor |
Bioorg Med Chem Lett 13: 931-5 (2003)
BindingDB Entry DOI: 10.7270/Q2HX1C1Z |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50099487
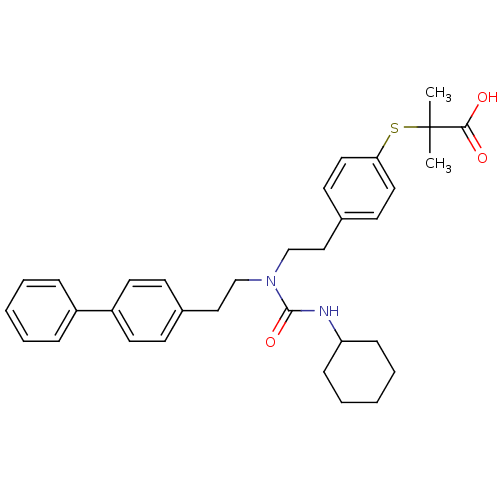
(2-(4-{2-[1-(2-Biphenyl-4-yl-ethyl)-3-cyclohexyl-ur...)Show SMILES CC(C)(Sc1ccc(CCN(CCc2ccc(cc2)-c2ccccc2)C(=O)NC2CCCCC2)cc1)C(O)=O Show InChI InChI=1S/C33H40N2O3S/c1-33(2,31(36)37)39-30-19-15-26(16-20-30)22-24-35(32(38)34-29-11-7-4-8-12-29)23-21-25-13-17-28(18-14-25)27-9-5-3-6-10-27/h3,5-6,9-10,13-20,29H,4,7-8,11-12,21-24H2,1-2H3,(H,34,38)(H,36,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
In vitro transcriptional activation in CV-1 cells expressing human Gal4-PPAR alpha ligand binding domain |
Bioorg Med Chem Lett 11: 1225-7 (2001)
BindingDB Entry DOI: 10.7270/Q2Z31XX0 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50360740
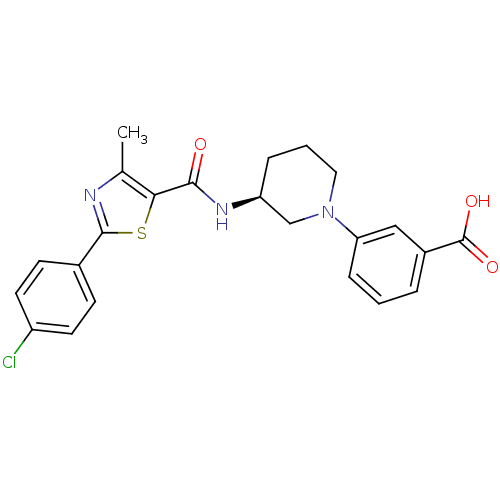
(CHEMBL1934476)Show SMILES Cc1nc(sc1C(=O)N[C@H]1CCCN(C1)c1cccc(c1)C(O)=O)-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C23H22ClN3O3S/c1-14-20(31-22(25-14)15-7-9-17(24)10-8-15)21(28)26-18-5-3-11-27(13-18)19-6-2-4-16(12-19)23(29)30/h2,4,6-10,12,18H,3,5,11,13H2,1H3,(H,26,28)(H,29,30)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Transactivation of human PPARalpha expressed in CHO-K1 cells |
Bioorg Med Chem Lett 22: 334-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.002
BindingDB Entry DOI: 10.7270/Q2G73F54 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50063093

(CHEMBL3398456)Show SMILES Cc1oc(nc1CCOc1cccc(CC[C@@H]2CN(C[C@@H]2C(O)=O)C(=O)Oc2ccccc2)c1)-c1ccccc1 |r| Show InChI InChI=1S/C32H32N2O6/c1-22-29(33-30(39-22)24-10-4-2-5-11-24)17-18-38-27-14-8-9-23(19-27)15-16-25-20-34(21-28(25)31(35)36)32(37)40-26-12-6-3-7-13-26/h2-14,19,25,28H,15-18,20-21H2,1H3,(H,35,36)/t25-,28+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development (R&D)
Curated by ChEMBL
| Assay Description
Agonist activity at GAL4 tagged human PPARalpha ligand binding domain expressed in HEK293 cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 25: 1196-205 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.066
BindingDB Entry DOI: 10.7270/Q20866ZP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50171996
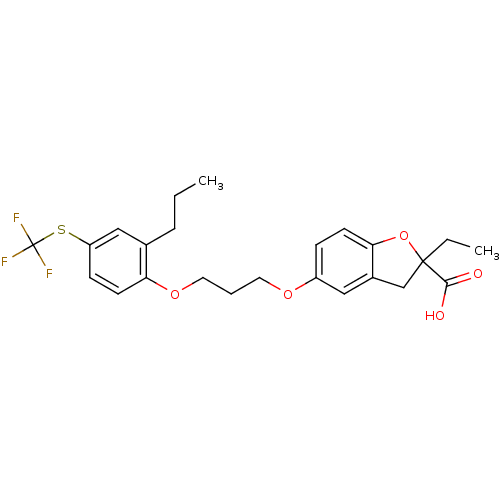
(2-Ethyl-5-[3-(2-propyl-4-trifluoromethylsulfanyl-p...)Show SMILES CCCc1cc(SC(F)(F)F)ccc1OCCCOc1ccc2OC(CC)(Cc2c1)C(O)=O Show InChI InChI=1S/C24H27F3O5S/c1-3-6-16-14-19(33-24(25,26)27)8-10-20(16)31-12-5-11-30-18-7-9-21-17(13-18)15-23(4-2,32-21)22(28)29/h7-10,13-14H,3-6,11-12,15H2,1-2H3,(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Factor Xa |
J Med Chem 48: 5589-99 (2005)
Article DOI: 10.1021/jm050373g
BindingDB Entry DOI: 10.7270/Q25T3K0M |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50194971
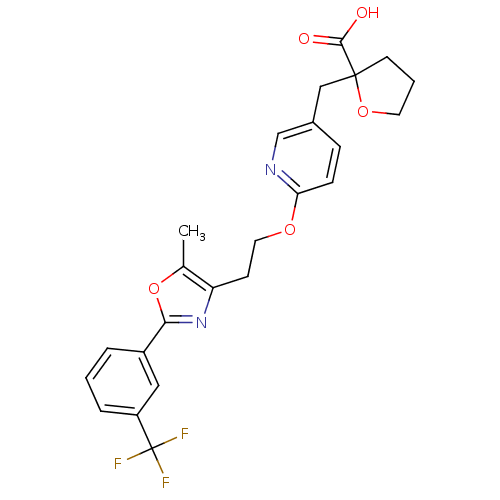
(2-((6-(2-(5-methyl-2-(3-(trifluoromethyl)phenyl)ox...)Show SMILES Cc1oc(nc1CCOc1ccc(CC2(CCCO2)C(O)=O)cn1)-c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C24H23F3N2O5/c1-15-19(29-21(34-15)17-4-2-5-18(12-17)24(25,26)27)8-11-32-20-7-6-16(14-28-20)13-23(22(30)31)9-3-10-33-23/h2,4-7,12,14H,3,8-11,13H2,1H3,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
Pfizer Global R&D
Curated by ChEMBL
| Assay Description
Agonist activity at human PPAR alpha in a HepG2 cells by PPAR-GAL4 transactivation assay |
Bioorg Med Chem Lett 16: 6120-3 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.110
BindingDB Entry DOI: 10.7270/Q20Z72XV |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50181911
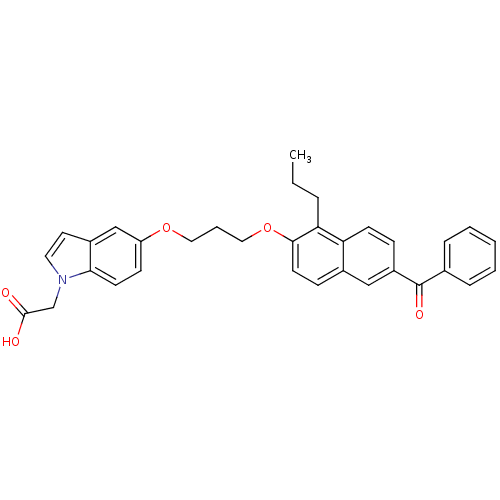
((5-{3-[(6-BENZOYL-1-PROPYL-2-NAPHTHYL)OXY]PROPOXY}...)Show SMILES CCCc1c(OCCCOc2ccc3n(CC(O)=O)ccc3c2)ccc2cc(ccc12)C(=O)c1ccccc1 Show InChI InChI=1S/C33H31NO5/c1-2-7-29-28-13-10-26(33(37)23-8-4-3-5-9-23)20-24(28)11-15-31(29)39-19-6-18-38-27-12-14-30-25(21-27)16-17-34(30)22-32(35)36/h3-5,8-17,20-21H,2,6-7,18-19,22H2,1H3,(H,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Activity at human PPAR alpha in Huh7 cells by transactivation assay |
J Med Chem 49: 6421-4 (2006)
Article DOI: 10.1021/jm060663c
BindingDB Entry DOI: 10.7270/Q2C53KG6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50214887

(2-(3-((benzo[d]oxazol-2-yl(3-(4-fluorophenoxy)prop...)Show SMILES CCC(Oc1cccc(CN(CCCOc2ccc(F)cc2)c2nc3ccccc3o2)c1)C(O)=O |w:2.1| Show InChI InChI=1S/C27H27FN2O5/c1-2-24(26(31)32)34-22-8-5-7-19(17-22)18-30(27-29-23-9-3-4-10-25(23)35-27)15-6-16-33-21-13-11-20(28)12-14-21/h3-5,7-14,17,24H,2,6,15-16,18H2,1H3,(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
Kowa Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant PPARalpha by GAL4 transactivation assay |
Bioorg Med Chem Lett 17: 4689-93 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.066
BindingDB Entry DOI: 10.7270/Q2J67GM5 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50246132

(2-methyl-2-(2-methyl-4-((1-methyl-5-(4-(pyrrolidin...)Show SMILES Cc1cc(CNC(=O)c2cc(-c3ccc(cc3)N3CCCC3)n(C)n2)ccc1OC(C)(C)C(O)=O Show InChI InChI=1S/C27H32N4O4/c1-18-15-19(7-12-24(18)35-27(2,3)26(33)34)17-28-25(32)22-16-23(30(4)29-22)20-8-10-21(11-9-20)31-13-5-6-14-31/h7-12,15-16H,5-6,13-14,17H2,1-4H3,(H,28,32)(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Agonist activity at human PPARalpha receptor by cell based transient transfection assay |
Bioorg Med Chem Lett 18: 6251-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.094
BindingDB Entry DOI: 10.7270/Q20Z733H |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50377303
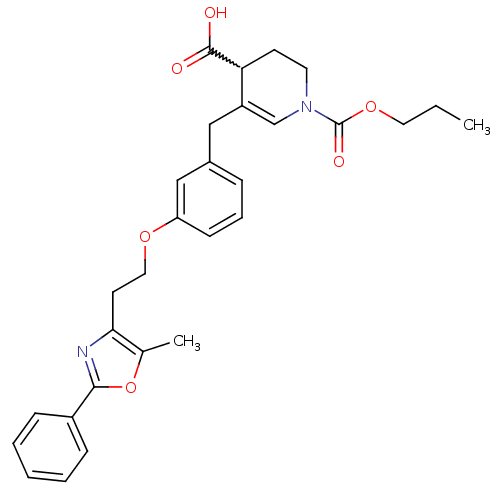
(CHEMBL256142)Show SMILES CCCOC(=O)N1CCC(C(O)=O)C(Cc2cccc(OCCc3nc(oc3C)-c3ccccc3)c2)=C1 |w:9.9,c:38| Show InChI InChI=1S/C29H32N2O6/c1-3-15-36-29(34)31-14-12-25(28(32)33)23(19-31)17-21-8-7-11-24(18-21)35-16-13-26-20(2)37-27(30-26)22-9-5-4-6-10-22/h4-11,18-19,25H,3,12-17H2,1-2H3,(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D
Curated by ChEMBL
| Assay Description
Agonist activity at human PPARgamma expressed in HEK293 cells assessed as luciferase activity by GAL4 transactivation assay |
Bioorg Med Chem Lett 18: 3545-50 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.014
BindingDB Entry DOI: 10.7270/Q2RX9D04 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50309072
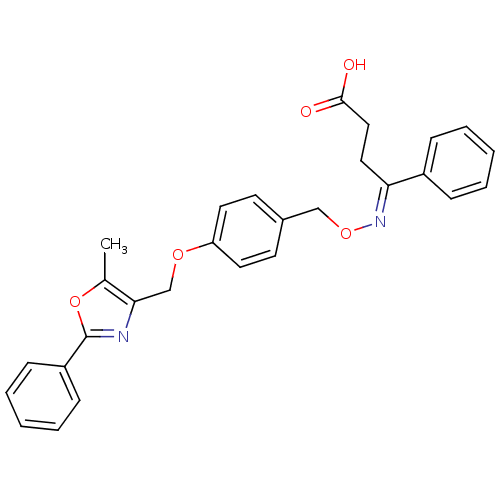
(CHEMBL592054 | IMIGLITAZAR)Show SMILES Cc1oc(nc1COc1ccc(CO\N=C(/CCC(O)=O)c2ccccc2)cc1)-c1ccccc1 Show InChI InChI=1S/C28H26N2O5/c1-20-26(29-28(35-20)23-10-6-3-7-11-23)19-33-24-14-12-21(13-15-24)18-34-30-25(16-17-27(31)32)22-8-4-2-5-9-22/h2-15H,16-19H2,1H3,(H,31,32)/b30-25+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Agonist activity at human PPARalpha transactivation expressed in human HePG2 cells |
Bioorg Med Chem Lett 18: 6471-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.062
BindingDB Entry DOI: 10.7270/Q2BK1D9Z |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50502539
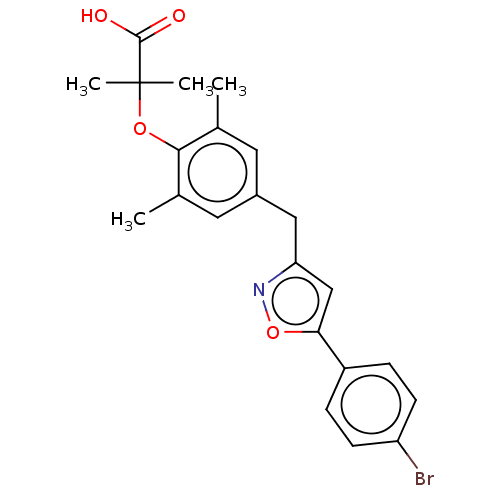
(CHEMBL4470753)Show SMILES Cc1cc(Cc2cc(on2)-c2ccc(Br)cc2)cc(C)c1OC(C)(C)C(O)=O Show InChI InChI=1S/C22H22BrNO4/c1-13-9-15(10-14(2)20(13)27-22(3,4)21(25)26)11-18-12-19(28-24-18)16-5-7-17(23)8-6-16/h5-10,12H,11H2,1-4H3,(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
WuXi AppTec (Shanghai) Co., Ltd
Curated by ChEMBL
| Assay Description
Agonist activity at PPARalpha (unknown origin) incubated for 3 to 16 hrs by pathhunter nuclear hormone receptor assay |
ACS Med Chem Lett 10: 1068-1073 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00189
BindingDB Entry DOI: 10.7270/Q2XK8JST |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50372112
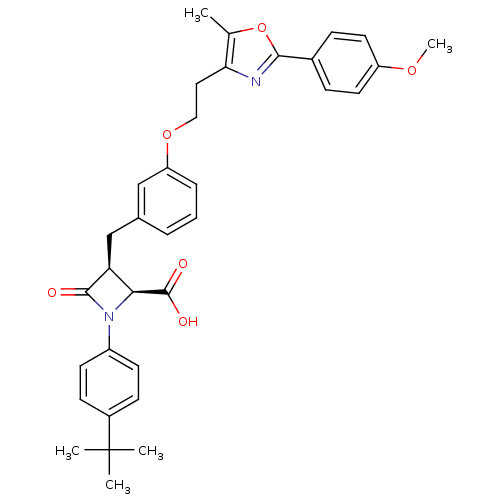
(CHEMBL257517)Show SMILES COc1ccc(cc1)-c1nc(CCOc2cccc(C[C@H]3[C@H](N(C3=O)c3ccc(cc3)C(C)(C)C)C(O)=O)c2)c(C)o1 Show InChI InChI=1S/C34H36N2O6/c1-21-29(35-31(42-21)23-9-15-26(40-5)16-10-23)17-18-41-27-8-6-7-22(19-27)20-28-30(33(38)39)36(32(28)37)25-13-11-24(12-14-25)34(2,3)4/h6-16,19,28,30H,17-18,20H2,1-5H3,(H,38,39)/t28-,30-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Agonist activity at PPARalpha in HEK293 cells by GAL4 transactivation assay |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50314814
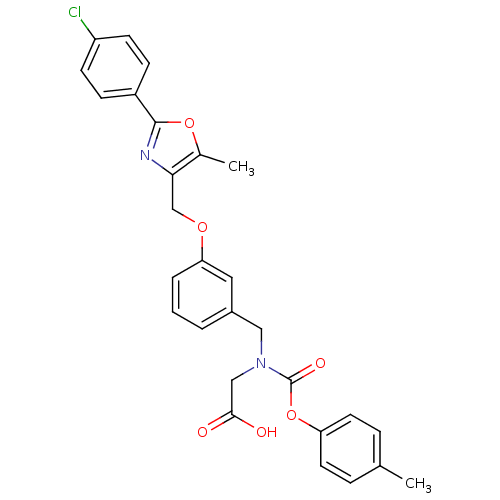
(2-((3-((2-(4-chlorophenyl)-5-methyloxazol-4-yl)met...)Show SMILES Cc1oc(nc1COc1cccc(CN(CC(O)=O)C(=O)Oc2ccc(C)cc2)c1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C28H25ClN2O6/c1-18-6-12-23(13-7-18)37-28(34)31(16-26(32)33)15-20-4-3-5-24(14-20)35-17-25-19(2)36-27(30-25)21-8-10-22(29)11-9-21/h3-14H,15-17H2,1-2H3,(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D
Curated by ChEMBL
| Assay Description
Agonist activity against human Gal4-fussed PPARalpha in HEK293 cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 20: 2933-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.019
BindingDB Entry DOI: 10.7270/Q2K35TS2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50124385
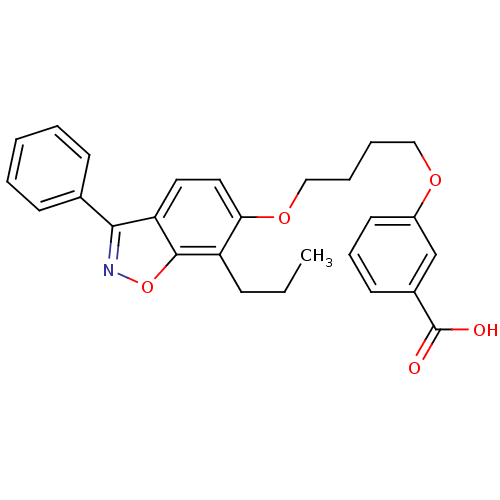
(3-[4-(3-Phenyl-7-propyl-benzo[d]isoxazol-6-yloxy)-...)Show SMILES CCCc1c(OCCCCOc2cccc(c2)C(O)=O)ccc2c(noc12)-c1ccccc1 Show InChI InChI=1S/C27H27NO5/c1-2-9-22-24(15-14-23-25(28-33-26(22)23)19-10-4-3-5-11-19)32-17-7-6-16-31-21-13-8-12-20(18-21)27(29)30/h3-5,8,10-15,18H,2,6-7,9,16-17H2,1H3,(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonistic activity was determined in COS1 cells transfected with GAL 4-PPAR alpha receptor |
Bioorg Med Chem Lett 13: 931-5 (2003)
BindingDB Entry DOI: 10.7270/Q2HX1C1Z |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50401644
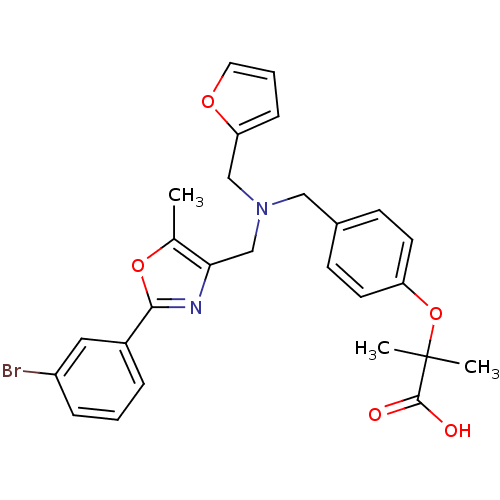
(CHEMBL2204600)Show SMILES Cc1oc(nc1CN(Cc1ccco1)Cc1ccc(OC(C)(C)C(O)=O)cc1)-c1cccc(Br)c1 Show InChI InChI=1S/C27H27BrN2O5/c1-18-24(29-25(34-18)20-6-4-7-21(28)14-20)17-30(16-23-8-5-13-33-23)15-19-9-11-22(12-10-19)35-27(2,3)26(31)32/h4-14H,15-17H2,1-3H3,(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Agonist activity at GAL4-fused PPARalpha assessed as transcriptional activity by cell based assay |
Bioorg Med Chem Lett 22: 7075-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.092
BindingDB Entry DOI: 10.7270/Q2HM59M5 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50214203

((+/-)-2-(3-((2-fluoro-4-(trifluoromethyl)benzamido...)Show SMILES CCC(Cc1ccc(OC)c(CNC(=O)c2ccc(cc2F)C(F)(F)F)c1)C(O)=O Show InChI InChI=1S/C21H21F4NO4/c1-3-13(20(28)29)8-12-4-7-18(30-2)14(9-12)11-26-19(27)16-6-5-15(10-17(16)22)21(23,24)25/h4-7,9-10,13H,3,8,11H2,1-2H3,(H,26,27)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by ChEMBL
| Assay Description
Effect on PPARalpha transactivation activity in HEK293 cells |
Bioorg Med Chem 15: 5177-90 (2007)
Article DOI: 10.1016/j.bmc.2007.05.023
BindingDB Entry DOI: 10.7270/Q2QZ29P7 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50189566
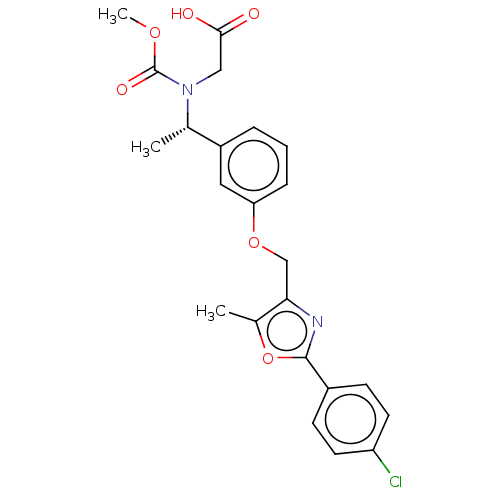
(CHEMBL3827820)Show SMILES COC(=O)N(CC(O)=O)[C@@H](C)c1cccc(OCc2nc(oc2C)-c2ccc(Cl)cc2)c1 |r| Show InChI InChI=1S/C23H23ClN2O6/c1-14(26(12-21(27)28)23(29)30-3)17-5-4-6-19(11-17)31-13-20-15(2)32-22(25-20)16-7-9-18(24)10-8-16/h4-11,14H,12-13H2,1-3H3,(H,27,28)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Agonist activity at Gal4-tagged human PPAR-alpha expressed in HEK cells by transactivation assay |
ACS Med Chem Lett 7: 590-4 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00033
BindingDB Entry DOI: 10.7270/Q2CR5W99 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50584469
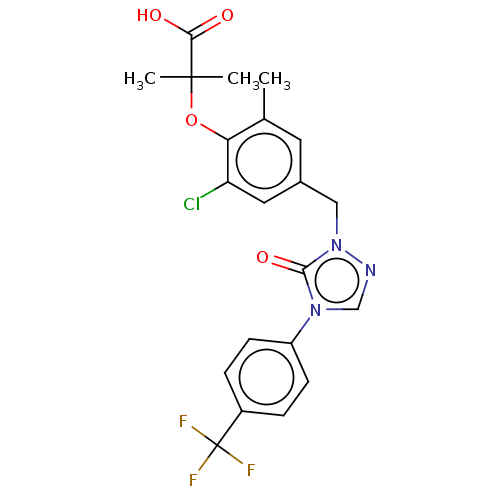
(CHEMBL5075951)Show SMILES Cc1cc(Cn2ncn(-c3ccc(cc3)C(F)(F)F)c2=O)cc(Cl)c1OC(C)(C)C(O)=O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at PPARalpha (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02002
BindingDB Entry DOI: 10.7270/Q2VQ36KV |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM539994
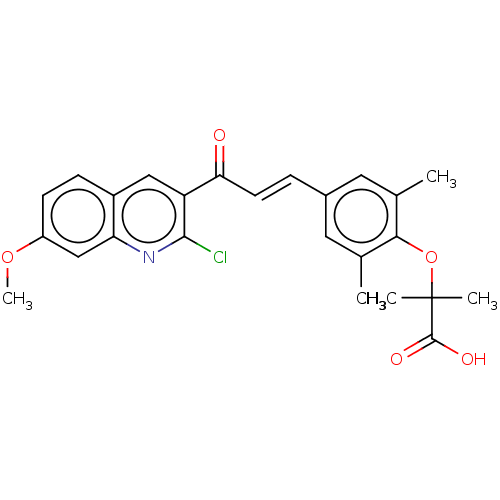
((E)-2-(4-(3-(2-chloro-7-methoxy-quinolin-3-yl)-3-o...)Show SMILES COc1ccc2cc(C(=O)\C=C\c3cc(C)c(OC(C)(C)C(O)=O)c(C)c3)c(Cl)nc2c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 8.62 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HEK293 cells were incubated in DMEM medium containing 10% of fetal bovine serum at 37° C. in the presence of 5% of CO2. 3×105/ml cells were plated in... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26H4MMP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50314829

(2-((3-((2-(4-isopropylphenyl)-5-methyloxazol-4-yl)...)Show SMILES CC(C)c1ccc(cc1)-c1nc(COc2cccc(CN(CC(O)=O)C(=O)Oc3ccc(C)cc3)c2)c(C)o1 Show InChI InChI=1S/C31H32N2O6/c1-20(2)24-10-12-25(13-11-24)30-32-28(22(4)38-30)19-37-27-7-5-6-23(16-27)17-33(18-29(34)35)31(36)39-26-14-8-21(3)9-15-26/h5-16,20H,17-19H2,1-4H3,(H,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Agonist activity at human PPARalpha LBD (167-468) expressed in HEK293 cells coexpressing GAL4-DBD by transactivation assay |
J Med Chem 53: 2854-64 (2010)
Article DOI: 10.1021/jm9016812
BindingDB Entry DOI: 10.7270/Q2B56JW0 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50344332
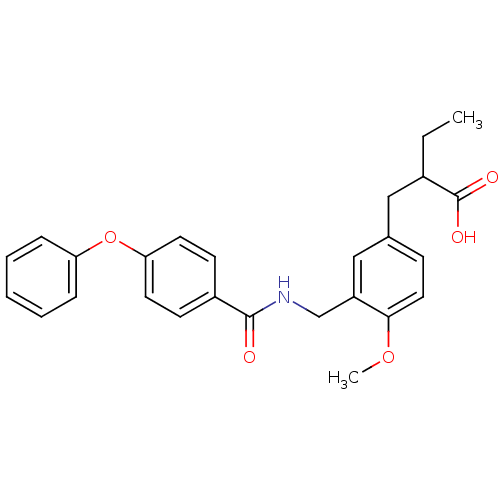
(2-(4-methoxy-3-((4-phenoxybenzamido)methyl)benzyl)...)Show SMILES CCC(Cc1ccc(OC)c(CNC(=O)c2ccc(Oc3ccccc3)cc2)c1)C(O)=O Show InChI InChI=1S/C26H27NO5/c1-3-19(26(29)30)15-18-9-14-24(31-2)21(16-18)17-27-25(28)20-10-12-23(13-11-20)32-22-7-5-4-6-8-22/h4-14,16,19H,3,15,17H2,1-2H3,(H,27,28)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a |
Okayama University
Curated by ChEMBL
| Assay Description
Agonist activity at Gal4-fussed PPARalpha expressed in human HEK293 cells by reporter gene assay |
Bioorg Med Chem 19: 3183-91 (2011)
Article DOI: 10.1016/j.bmc.2011.03.064
BindingDB Entry DOI: 10.7270/Q2S75GNC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50314814

(2-((3-((2-(4-chlorophenyl)-5-methyloxazol-4-yl)met...)Show SMILES Cc1oc(nc1COc1cccc(CN(CC(O)=O)C(=O)Oc2ccc(C)cc2)c1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C28H25ClN2O6/c1-18-6-12-23(13-7-18)37-28(34)31(16-26(32)33)15-20-4-3-5-24(14-20)35-17-25-19(2)36-27(30-25)21-8-10-22(29)11-9-21/h3-14H,15-17H2,1-2H3,(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Agonist activity at human PPARalpha LBD (167-468) expressed in HEK293 cells coexpressing GAL4-DBD by transactivation assay |
J Med Chem 53: 2854-64 (2010)
Article DOI: 10.1021/jm9016812
BindingDB Entry DOI: 10.7270/Q2B56JW0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50486807
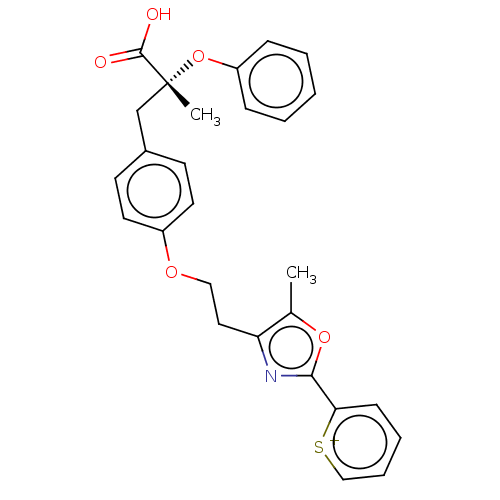
(CHEMBL2237301)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@@](C)(Oc2ccccc2)C(O)=O)cc1)-c1cccc[s+]1 |r| Show InChI InChI=1S/C27H25NO5S/c1-19-23(28-25(32-19)24-10-6-7-17-34-24)15-16-31-21-13-11-20(12-14-21)18-27(2,26(29)30)33-22-8-4-3-5-9-22/h3-14,17H,15-16,18H2,1-2H3/p+1/t27-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
| n/a | n/a | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Transactivation of Homo sapiens (human) PPARalpha assessed as luciferase activity by reporter gene assay |
Citation and Details
Article DOI: 10.1007/s00044-011-9818-7
BindingDB Entry DOI: 10.7270/Q2R49TNP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50145721
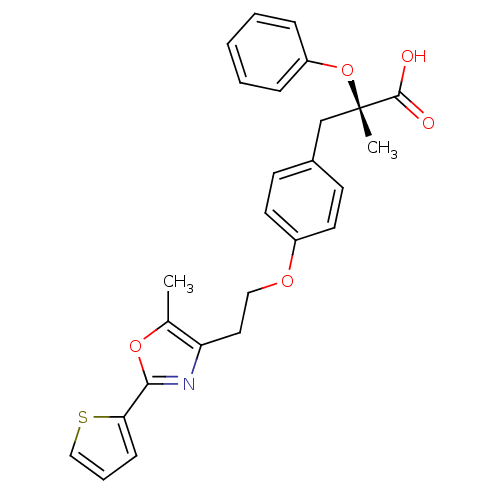
((S)-2-Methyl-3-{4-[2-(5-methyl-2-thiophen-2-yl-oxa...)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@](C)(Oc2ccccc2)C(O)=O)cc1)-c1cccs1 Show InChI InChI=1S/C26H25NO5S/c1-18-22(27-24(31-18)23-9-6-16-33-23)14-15-30-20-12-10-19(11-13-20)17-26(2,25(28)29)32-21-7-4-3-5-8-21/h3-13,16H,14-15,17H2,1-2H3,(H,28,29)/t26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Transactivation of Homo sapiens (human) PPARalpha assessed as luciferase activity by reporter gene assay |
Citation and Details
Article DOI: 10.1007/s00044-011-9818-7
BindingDB Entry DOI: 10.7270/Q2R49TNP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50099491
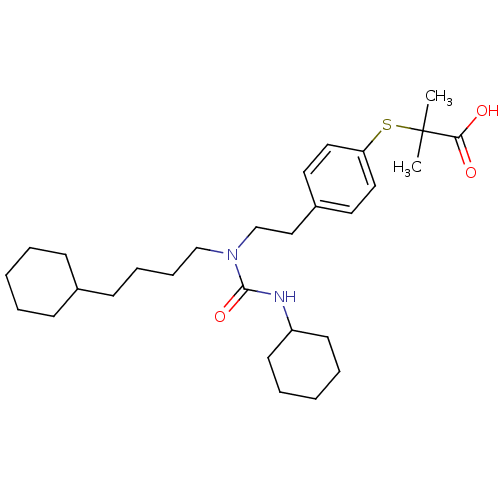
(2-(4-(2-(3-cyclohexyl-1-(4-cyclohexylbutyl)ureido)...)Show SMILES CC(C)(Sc1ccc(CCN(CCCCC2CCCCC2)C(=O)NC2CCCCC2)cc1)C(O)=O Show InChI InChI=1S/C29H46N2O3S/c1-29(2,27(32)33)35-26-18-16-24(17-19-26)20-22-31(28(34)30-25-14-7-4-8-15-25)21-10-9-13-23-11-5-3-6-12-23/h16-19,23,25H,3-15,20-22H2,1-2H3,(H,30,34)(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116615
BindingDB Entry DOI: 10.7270/Q2DJ5KQ6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50099493

(2-(4-{2-[1-(2-Biphenyl-4-yl-ethyl)-3-(2,4,5-trimet...)Show SMILES Cc1cc(C)c(NC(=O)N(CCc2ccc(SC(C)(C)C(O)=O)cc2)CCc2ccc(cc2)-c2ccccc2)cc1C Show InChI InChI=1S/C36H40N2O3S/c1-25-23-27(3)33(24-26(25)2)37-35(41)38(21-19-28-11-15-31(16-12-28)30-9-7-6-8-10-30)22-20-29-13-17-32(18-14-29)42-36(4,5)34(39)40/h6-18,23-24H,19-22H2,1-5H3,(H,37,41)(H,39,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
In vitro transcriptional activation in CV-1 cells expressing human Gal4-PPAR alpha ligand binding domain |
Bioorg Med Chem Lett 11: 1225-7 (2001)
BindingDB Entry DOI: 10.7270/Q2Z31XX0 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50580803
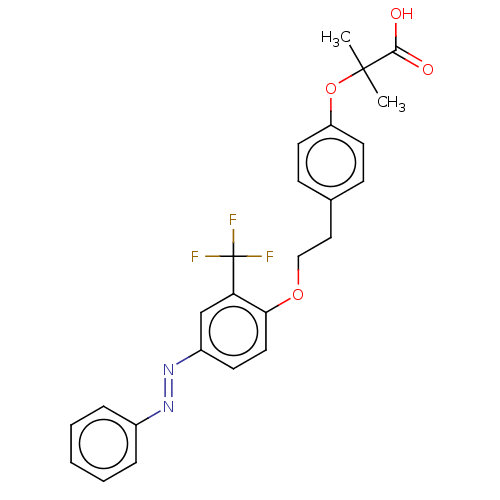
(CHEMBL5075710)Show SMILES CC(C)(Oc1ccc(CCOc2ccc(cc2C(F)(F)F)\N=N\c2ccccc2)cc1)C(O)=O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at Gal4-fused human PPARalpha LBD transfected in HEK293T cells assessed as transcriptional activation incubated for 14 to 16 hrs by ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00810
BindingDB Entry DOI: 10.7270/Q2N87FNT |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50099491

(2-(4-(2-(3-cyclohexyl-1-(4-cyclohexylbutyl)ureido)...)Show SMILES CC(C)(Sc1ccc(CCN(CCCCC2CCCCC2)C(=O)NC2CCCCC2)cc1)C(O)=O Show InChI InChI=1S/C29H46N2O3S/c1-29(2,27(32)33)35-26-18-16-24(17-19-26)20-22-31(28(34)30-25-14-7-4-8-15-25)21-10-9-13-23-11-5-3-6-12-23/h16-19,23,25H,3-15,20-22H2,1-2H3,(H,30,34)(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human PPARalpha transfected in human HepG2 cells incubated for 18 hrs by Renilla/Firefly dual-luciferase reporter assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113807
BindingDB Entry DOI: 10.7270/Q2KW5KV0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50099488

(2-(4-{2-[3-(3-Cyano-phenyl)-1-(4-cyclohexyl-butyl)...)Show SMILES CC(C)(Sc1ccc(CCN(CCCCC2CCCCC2)C(=O)Nc2cccc(c2)C#N)cc1)C(O)=O Show InChI InChI=1S/C30H39N3O3S/c1-30(2,28(34)35)37-27-16-14-24(15-17-27)18-20-33(19-7-6-11-23-9-4-3-5-10-23)29(36)32-26-13-8-12-25(21-26)22-31/h8,12-17,21,23H,3-7,9-11,18-20H2,1-2H3,(H,32,36)(H,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
In vitro transcriptional activation in CV-1 cells expressing human Gal4-PPAR alpha ligand binding domain |
Bioorg Med Chem Lett 11: 1225-7 (2001)
BindingDB Entry DOI: 10.7270/Q2Z31XX0 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50145721

((S)-2-Methyl-3-{4-[2-(5-methyl-2-thiophen-2-yl-oxa...)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@](C)(Oc2ccccc2)C(O)=O)cc1)-c1cccs1 Show InChI InChI=1S/C26H25NO5S/c1-18-22(27-24(31-18)23-9-6-16-33-23)14-15-30-20-12-10-19(11-13-20)17-26(2,25(28)29)32-21-7-4-3-5-8-21/h3-13,16H,14-15,17H2,1-2H3,(H,28,29)/t26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Transactivation of GAL4-fused Homo sapiens (human) PPARalpha DNA binding domain expressed in African green monkey CV1 cells by luciferase reporter ge... |
Citation and Details
Article DOI: 10.1007/s00044-012-0003-4
BindingDB Entry DOI: 10.7270/Q280544F |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50145721

((S)-2-Methyl-3-{4-[2-(5-methyl-2-thiophen-2-yl-oxa...)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@](C)(Oc2ccccc2)C(O)=O)cc1)-c1cccs1 Show InChI InChI=1S/C26H25NO5S/c1-18-22(27-24(31-18)23-9-6-16-33-23)14-15-30-20-12-10-19(11-13-20)17-26(2,25(28)29)32-21-7-4-3-5-8-21/h3-13,16H,14-15,17H2,1-2H3,(H,28,29)/t26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Transactivation of GAL4-fused Homo sapiens (human) PPARalpha DNA binding domain expressed in African green monkey CV1 cells by luciferase reporter ge... |
Citation and Details
Article DOI: 10.1007/s00044-012-0003-4
BindingDB Entry DOI: 10.7270/Q280544F |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50377321

(CHEMBL256358)Show SMILES CC(C)COC(=O)N1CCC(C(O)=O)C(Cc2cccc(OCCc3nc(oc3C)-c3ccccc3)c2)=C1 |w:10.10,c:39| Show InChI InChI=1S/C30H34N2O6/c1-20(2)19-37-30(35)32-14-12-26(29(33)34)24(18-32)16-22-8-7-11-25(17-22)36-15-13-27-21(3)38-28(31-27)23-9-5-4-6-10-23/h4-11,17-18,20,26H,12-16,19H2,1-3H3,(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D
Curated by ChEMBL
| Assay Description
Agonist activity at human PPARgamma expressed in HEK293 cells assessed as luciferase activity by GAL4 transactivation assay |
Bioorg Med Chem Lett 18: 3545-50 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.014
BindingDB Entry DOI: 10.7270/Q2RX9D04 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50401640
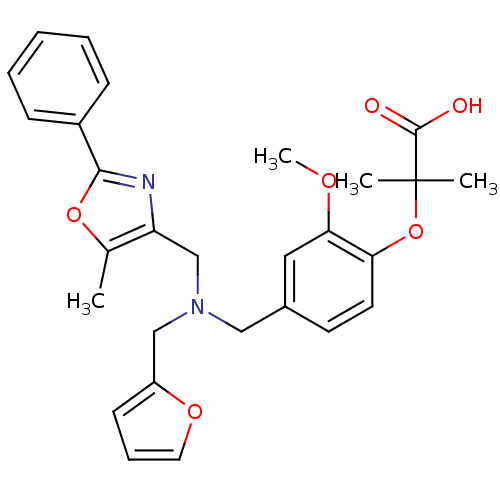
(CHEMBL2204596)Show SMILES COc1cc(CN(Cc2ccco2)Cc2nc(oc2C)-c2ccccc2)ccc1OC(C)(C)C(O)=O Show InChI InChI=1S/C28H30N2O6/c1-19-23(29-26(35-19)21-9-6-5-7-10-21)18-30(17-22-11-8-14-34-22)16-20-12-13-24(25(15-20)33-4)36-28(2,3)27(31)32/h5-15H,16-18H2,1-4H3,(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Agonist activity at GAL4-fused PPARalpha assessed as transcriptional activity by cell based assay |
Bioorg Med Chem Lett 22: 7075-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.092
BindingDB Entry DOI: 10.7270/Q2HM59M5 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50294693

((S)-3-(4-(2-(6-((cyclopropylmethoxyimino)(phenyl)m...)Show SMILES CCO[C@@H](Cc1ccc(OCCN2CCC(C)(C)c3cc(ccc23)C(=N\OCC2CC2)\c2ccccc2)cc1)C(O)=O |r| Show InChI InChI=1S/C35H42N2O5/c1-4-40-32(34(38)39)22-25-12-15-29(16-13-25)41-21-20-37-19-18-35(2,3)30-23-28(14-17-31(30)37)33(27-8-6-5-7-9-27)36-42-24-26-10-11-26/h5-9,12-17,23,26,32H,4,10-11,18-22,24H2,1-3H3,(H,38,39)/b36-33+/t32-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
UMR GICC
Curated by ChEMBL
| Assay Description
Agonist activity at human PPARalpha by Gal4 transactivation assay |
Bioorg Med Chem Lett 19: 2683-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.143
BindingDB Entry DOI: 10.7270/Q2KP826B |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50214894
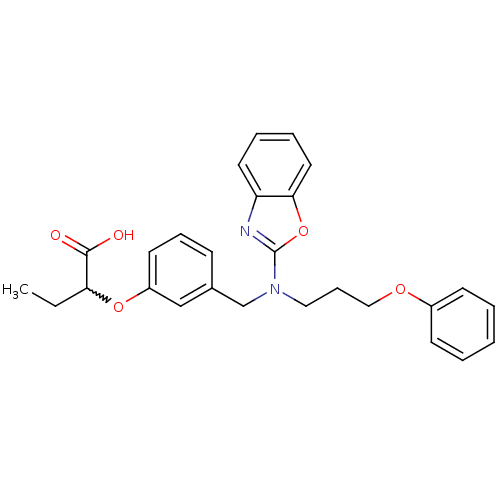
(2-(3-((benzo[d]oxazol-2-yl(3-phenoxypropyl)amino)m...)Show SMILES CCC(Oc1cccc(CN(CCCOc2ccccc2)c2nc3ccccc3o2)c1)C(O)=O |w:2.2| Show InChI InChI=1S/C27H28N2O5/c1-2-24(26(30)31)33-22-13-8-10-20(18-22)19-29(16-9-17-32-21-11-4-3-5-12-21)27-28-23-14-6-7-15-25(23)34-27/h3-8,10-15,18,24H,2,9,16-17,19H2,1H3,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
Kowa Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant PPARalpha by GAL4 transactivation assay |
Bioorg Med Chem Lett 17: 4689-93 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.066
BindingDB Entry DOI: 10.7270/Q2J67GM5 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50168560

((R)-7-[3-(2-Chloro-4-cyclopentyl-phenoxy)-propoxy]...)Show SMILES C[C@@]1(CCc2ccc(OCCCOc3ccc(cc3Cl)C3CCCC3)cc2O1)C(O)=O Show InChI InChI=1S/C25H29ClO5/c1-25(24(27)28)12-11-18-7-9-20(16-23(18)31-25)29-13-4-14-30-22-10-8-19(15-21(22)26)17-5-2-3-6-17/h7-10,15-17H,2-6,11-14H2,1H3,(H,27,28)/t25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity against human PPARalpha in COS-1 cell Gal4 assay |
Bioorg Med Chem Lett 15: 3347-51 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.028
BindingDB Entry DOI: 10.7270/Q22N51TV |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50171991
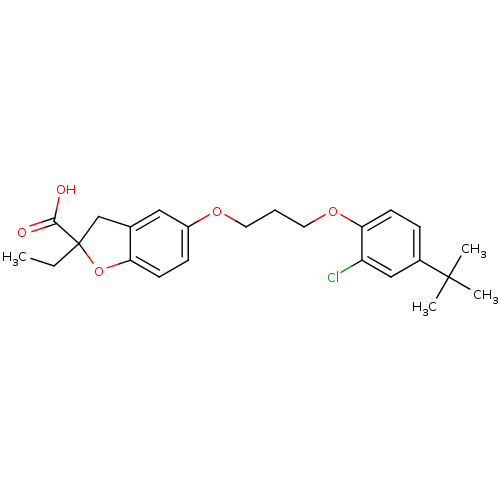
(5-[3-(4-tert-Butyl-2-chloro-phenoxy)-propoxy]-2-et...)Show SMILES CCC1(Cc2cc(OCCCOc3ccc(cc3Cl)C(C)(C)C)ccc2O1)C(O)=O Show InChI InChI=1S/C24H29ClO5/c1-5-24(22(26)27)15-16-13-18(8-10-20(16)30-24)28-11-6-12-29-21-9-7-17(14-19(21)25)23(2,3)4/h7-10,13-14H,5-6,11-12,15H2,1-4H3,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Effective concentration against human PPAR-alpha in Gal4 transactivation assay |
J Med Chem 48: 5589-99 (2005)
Article DOI: 10.1021/jm050373g
BindingDB Entry DOI: 10.7270/Q25T3K0M |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50145721

((S)-2-Methyl-3-{4-[2-(5-methyl-2-thiophen-2-yl-oxa...)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@](C)(Oc2ccccc2)C(O)=O)cc1)-c1cccs1 Show InChI InChI=1S/C26H25NO5S/c1-18-22(27-24(31-18)23-9-6-16-33-23)14-15-30-20-12-10-19(11-13-20)17-26(2,25(28)29)32-21-7-4-3-5-8-21/h3-13,16H,14-15,17H2,1-2H3,(H,28,29)/t26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Transcriptional activation of reporter assay by human Peroxisome proliferator activated receptor alpha in CV-1 cells |
J Med Chem 47: 2422-5 (2004)
Article DOI: 10.1021/jm0342616
BindingDB Entry DOI: 10.7270/Q2TQ6100 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50145710
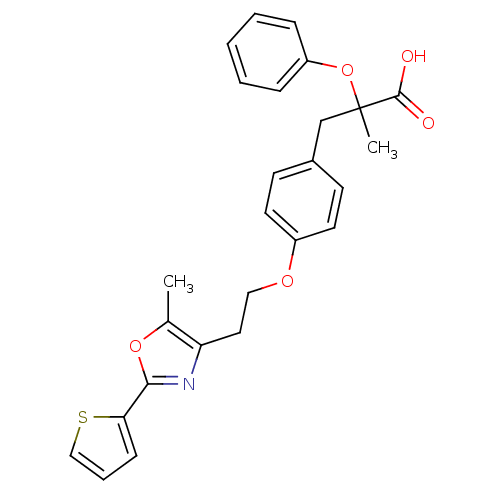
(2-Methyl-3-{4-[2-(5-methyl-2-thiophen-2-yl-oxazol-...)Show SMILES Cc1oc(nc1CCOc1ccc(CC(C)(Oc2ccccc2)C(O)=O)cc1)-c1cccs1 Show InChI InChI=1S/C26H25NO5S/c1-18-22(27-24(31-18)23-9-6-16-33-23)14-15-30-20-12-10-19(11-13-20)17-26(2,25(28)29)32-21-7-4-3-5-8-21/h3-13,16H,14-15,17H2,1-2H3,(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Effective concentration against human peroxisome proliferator activated receptor alpha in Gal4 transactivation assay |
J Med Chem 47: 4118-27 (2004)
Article DOI: 10.1021/jm030631e
BindingDB Entry DOI: 10.7270/Q2DR2TZ5 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50314823

(2-((3-((5-methyl-2-p-tolyloxazol-4-yl)methoxy)benz...)Show SMILES Cc1oc(nc1COc1cccc(CN(CC(O)=O)C(=O)Oc2ccc(C)cc2)c1)-c1ccc(C)cc1 Show InChI InChI=1S/C29H28N2O6/c1-19-7-11-23(12-8-19)28-30-26(21(3)36-28)18-35-25-6-4-5-22(15-25)16-31(17-27(32)33)29(34)37-24-13-9-20(2)10-14-24/h4-15H,16-18H2,1-3H3,(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Agonist activity at human PPARalpha LBD (167-468) expressed in HEK293 cells coexpressing GAL4-DBD by transactivation assay |
J Med Chem 53: 2854-64 (2010)
Article DOI: 10.1021/jm9016812
BindingDB Entry DOI: 10.7270/Q2B56JW0 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50314831
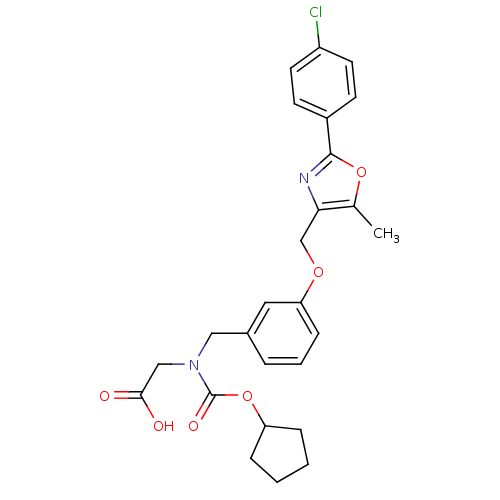
(2-((3-((2-(4-chlorophenyl)-5-methyloxazol-4-yl)met...)Show SMILES Cc1oc(nc1COc1cccc(CN(CC(O)=O)C(=O)OC2CCCC2)c1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C26H27ClN2O6/c1-17-23(28-25(34-17)19-9-11-20(27)12-10-19)16-33-22-8-4-5-18(13-22)14-29(15-24(30)31)26(32)35-21-6-2-3-7-21/h4-5,8-13,21H,2-3,6-7,14-16H2,1H3,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 9.20 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Agonist activity at human PPARalpha LBD (167-468) expressed in HEK293 cells coexpressing GAL4-DBD by transactivation assay |
J Med Chem 53: 2854-64 (2010)
Article DOI: 10.1021/jm9016812
BindingDB Entry DOI: 10.7270/Q2B56JW0 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50584470
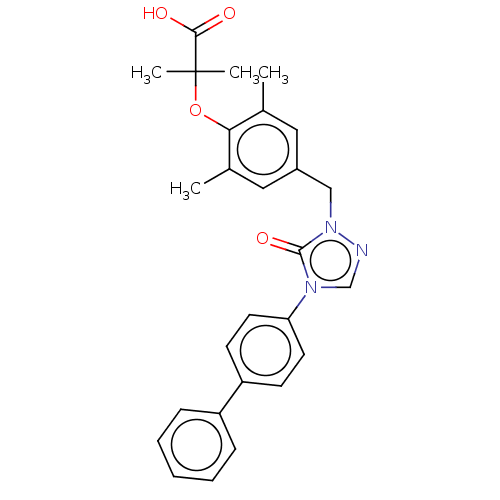
(CHEMBL5087638)Show SMILES Cc1cc(Cn2ncn(-c3ccc(cc3)-c3ccccc3)c2=O)cc(C)c1OC(C)(C)C(O)=O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at PPARalpha (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02002
BindingDB Entry DOI: 10.7270/Q2VQ36KV |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM28800
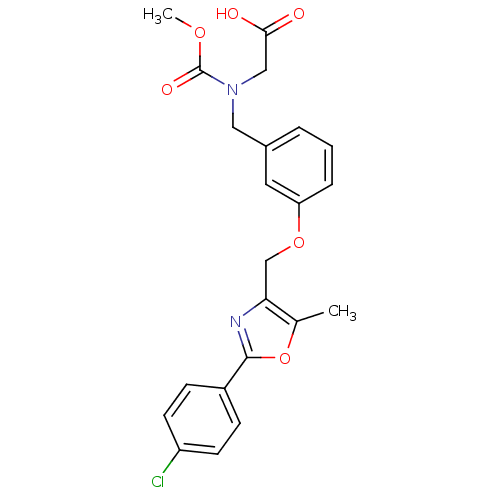
(2-{[(3-{[2-(4-chlorophenyl)-5-methyl-1,3-oxazol-4-...)Show SMILES COC(=O)N(CC(O)=O)Cc1cccc(OCc2nc(oc2C)-c2ccc(Cl)cc2)c1 Show InChI InChI=1S/C22H21ClN2O6/c1-14-19(24-21(31-14)16-6-8-17(23)9-7-16)13-30-18-5-3-4-15(10-18)11-25(12-20(26)27)22(28)29-2/h3-10H,11-13H2,1-2H3,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D
Curated by ChEMBL
| Assay Description
Agonist activity against human Gal4-fussed PPARalpha in HEK293 cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 20: 2933-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.019
BindingDB Entry DOI: 10.7270/Q2K35TS2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50502541
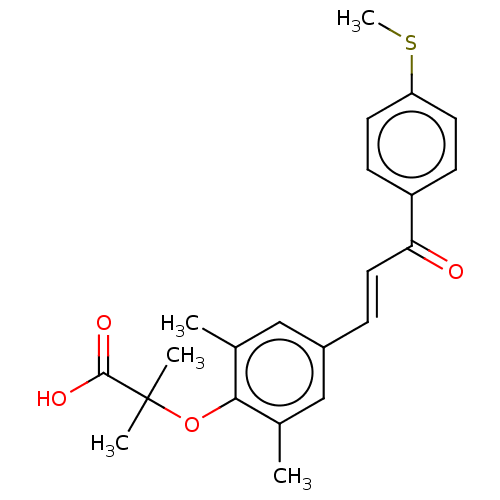
(Elafibranor | GFT-505 | Gft505)Show SMILES CSc1ccc(cc1)C(=O)\C=C\c1cc(C)c(OC(C)(C)C(O)=O)c(C)c1 Show InChI InChI=1S/C22H24O4S/c1-14-12-16(13-15(2)20(14)26-22(3,4)21(24)25)6-11-19(23)17-7-9-18(27-5)10-8-17/h6-13H,1-5H3,(H,24,25)/b11-6+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at PPARalpha (unknown origin) |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data