

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM50253810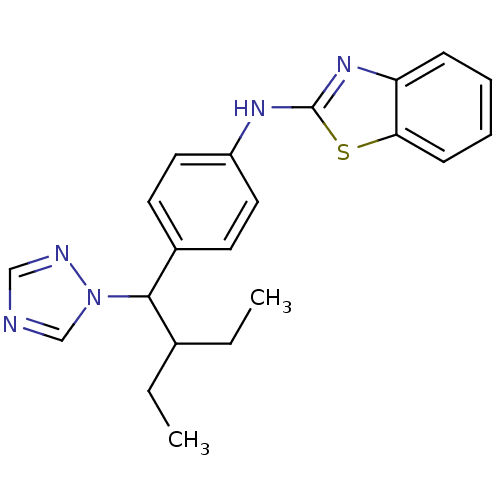 (CHEMBL459505 | N-(4-(2-ethyl-1-(1H-1,2,4-triazol-1...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Eighteen compounds were tested as potential inhibitors of CYP26A1 and CYP26B1. The formation of 9-cis-4-OH-RA metabolite was monitored and the percen... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM393281 (US9963439, R116010) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Eighteen compounds were tested as potential inhibitors of CYP26A1 and CYP26B1. The formation of 9-cis-4-OH-RA metabolite was monitored and the percen... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26A1 (Homo sapiens (Human)) | BDBM393281 (US9963439, R116010) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Eighteen compounds were tested as potential inhibitors of CYP26A1 and CYP26B1. The formation of 9-cis-4-OH-RA metabolite was monitored and the percen... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26A1 (Homo sapiens (Human)) | BDBM50253810 (CHEMBL459505 | N-(4-(2-ethyl-1-(1H-1,2,4-triazol-1...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Eighteen compounds were tested as potential inhibitors of CYP26A1 and CYP26B1. The formation of 9-cis-4-OH-RA metabolite was monitored and the percen... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM50157605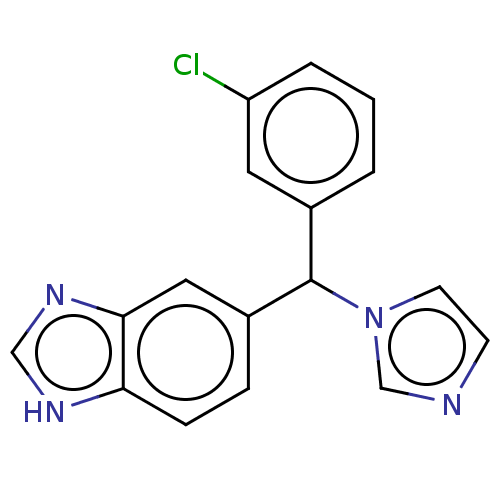 (Liarozole | Liazal | R-75251 | US9963439, Liarozol...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Eighteen compounds were tested as potential inhibitors of CYP26A1 and CYP26B1. The formation of 9-cis-4-OH-RA metabolite was monitored and the percen... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM151585 (US11739089, Compound Ketoconazole | US8987315, Ket...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid PDB UniChem Similars | DrugBank PDB US Patent | n/a | n/a | <20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM393281 (US9963439, R116010) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM393290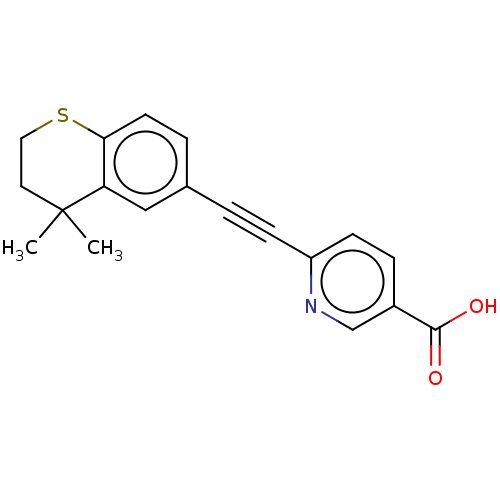 (DDBEP | US9963439, Tazarotenic acid) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM151585 (US11739089, Compound Ketoconazole | US8987315, Ket...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Eighteen compounds were tested as potential inhibitors of CYP26A1 and CYP26B1. The formation of 9-cis-4-OH-RA metabolite was monitored and the percen... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50253810 (CHEMBL459505 | N-(4-(2-ethyl-1-(1H-1,2,4-triazol-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50253810 (CHEMBL459505 | N-(4-(2-ethyl-1-(1H-1,2,4-triazol-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50157605 (Liarozole | Liazal | R-75251 | US9963439, Liarozol...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26A1 (Homo sapiens (Human)) | BDBM151585 (US11739089, Compound Ketoconazole | US8987315, Ket...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid PDB UniChem Similars | DrugBank US Patent | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Eighteen compounds were tested as potential inhibitors of CYP26A1 and CYP26B1. The formation of 9-cis-4-OH-RA metabolite was monitored and the percen... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50253810 (CHEMBL459505 | N-(4-(2-ethyl-1-(1H-1,2,4-triazol-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50157724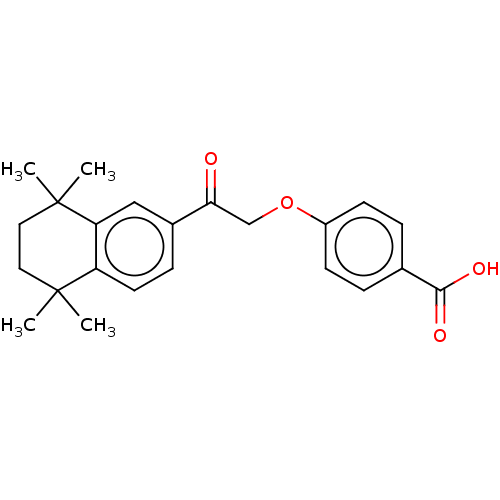 (CHEMBL3787564 | US9963439, Compound B) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50265920 (4-((5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthal...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | US Patent | n/a | n/a | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM393295 (US9963439, Compound F) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM151585 (US11739089, Compound Ketoconazole | US8987315, Ket...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid PDB UniChem Similars | DrugBank US Patent | n/a | n/a | 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM393294 (US9963439, Compound E) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50157605 (Liarozole | Liazal | R-75251 | US9963439, Liarozol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50157731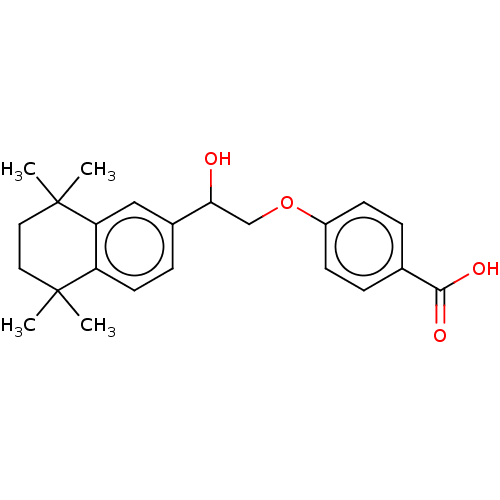 (CHEMBL3785511 | US9963439, Compound C) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 1.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM393281 (US9963439, R116010) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26A1 (Homo sapiens (Human)) | BDBM50157605 (Liarozole | Liazal | R-75251 | US9963439, Liarozol...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Eighteen compounds were tested as potential inhibitors of CYP26A1 and CYP26B1. The formation of 9-cis-4-OH-RA metabolite was monitored and the percen... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50052414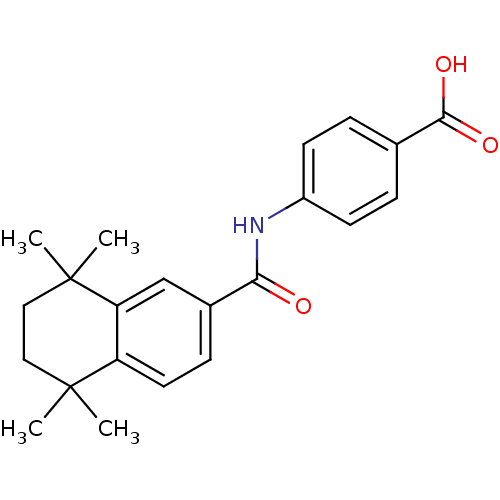 (4-(1,1,4,4-tetramethyl-1,2,3,4-tetrahydronaphthale...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM151585 (US11739089, Compound Ketoconazole | US8987315, Ket...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid PDB UniChem Similars | DrugBank US Patent | n/a | n/a | 2.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM393287 (US9963439, Compound D) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM393293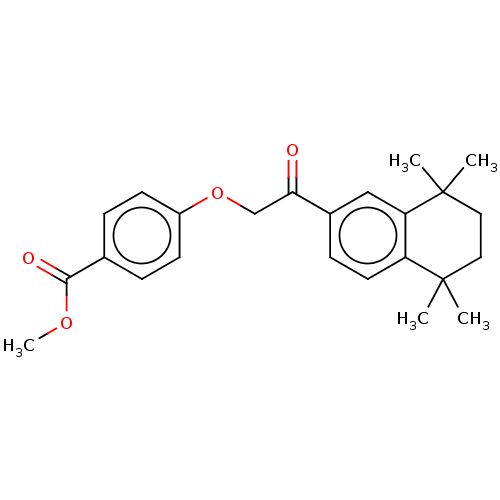 (US9963439, Compound A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50265951 (CHEMBL1657 | TAZAROTENE | US9963439, Tazarotene | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | US Patent | n/a | n/a | 3.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26A1 (Homo sapiens (Human)) | BDBM50157731 (CHEMBL3785511 | US9963439, Compound C) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 3.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Eighteen compounds were tested as potential inhibitors of CYP26A1 and CYP26B1. The formation of 9-cis-4-OH-RA metabolite was monitored and the percen... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50032219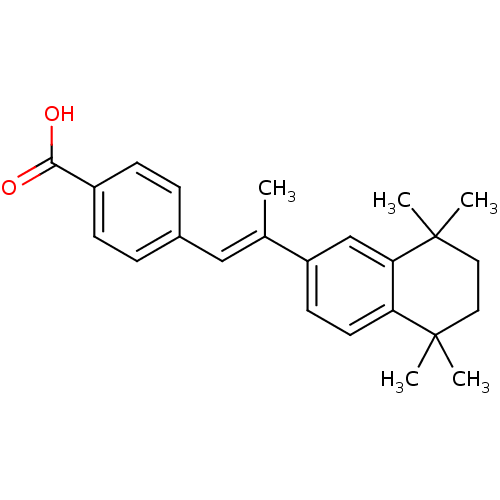 ((E)-4-(2-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydrona...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26A1 (Homo sapiens (Human)) | BDBM393287 (US9963439, Compound D) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Eighteen compounds were tested as potential inhibitors of CYP26A1 and CYP26B1. The formation of 9-cis-4-OH-RA metabolite was monitored and the percen... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26A1 (Homo sapiens (Human)) | BDBM50157724 (CHEMBL3787564 | US9963439, Compound B) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Eighteen compounds were tested as potential inhibitors of CYP26A1 and CYP26B1. The formation of 9-cis-4-OH-RA metabolite was monitored and the percen... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26A1 (Homo sapiens (Human)) | BDBM50032219 ((E)-4-(2-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydrona...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Eighteen compounds were tested as potential inhibitors of CYP26A1 and CYP26B1. The formation of 9-cis-4-OH-RA metabolite was monitored and the percen... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM393281 (US9963439, R116010) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26A1 (Homo sapiens (Human)) | BDBM50052414 (4-(1,1,4,4-tetramethyl-1,2,3,4-tetrahydronaphthale...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Eighteen compounds were tested as potential inhibitors of CYP26A1 and CYP26B1. The formation of 9-cis-4-OH-RA metabolite was monitored and the percen... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26A1 (Homo sapiens (Human)) | BDBM393290 (DDBEP | US9963439, Tazarotenic acid) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Eighteen compounds were tested as potential inhibitors of CYP26A1 and CYP26B1. The formation of 9-cis-4-OH-RA metabolite was monitored and the percen... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26A1 (Homo sapiens (Human)) | BDBM393295 (US9963439, Compound F) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Eighteen compounds were tested as potential inhibitors of CYP26A1 and CYP26B1. The formation of 9-cis-4-OH-RA metabolite was monitored and the percen... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50157605 (Liarozole | Liazal | R-75251 | US9963439, Liarozol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26A1 (Homo sapiens (Human)) | BDBM50265920 (4-((5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthal...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | US Patent | n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Eighteen compounds were tested as potential inhibitors of CYP26A1 and CYP26B1. The formation of 9-cis-4-OH-RA metabolite was monitored and the percen... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50061625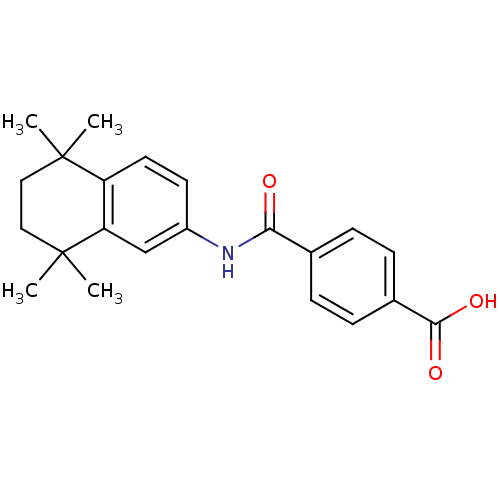 (4-[(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthal...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26A1 (Homo sapiens (Human)) | BDBM50061625 (4-[(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthal...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Eighteen compounds were tested as potential inhibitors of CYP26A1 and CYP26B1. The formation of 9-cis-4-OH-RA metabolite was monitored and the percen... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26A1 (Homo sapiens (Human)) | BDBM393289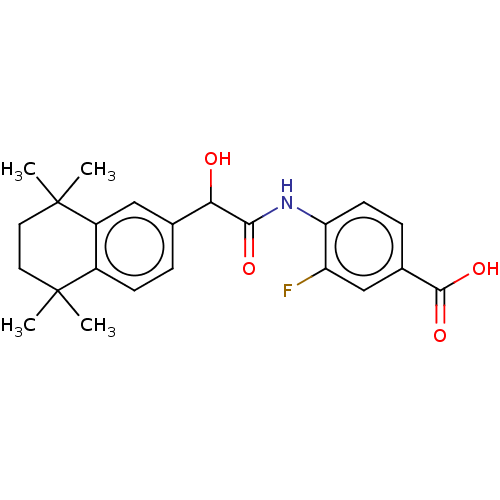 (US9963439, BMS961) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 1.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Eighteen compounds were tested as potential inhibitors of CYP26A1 and CYP26B1. The formation of 9-cis-4-OH-RA metabolite was monitored and the percen... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM36810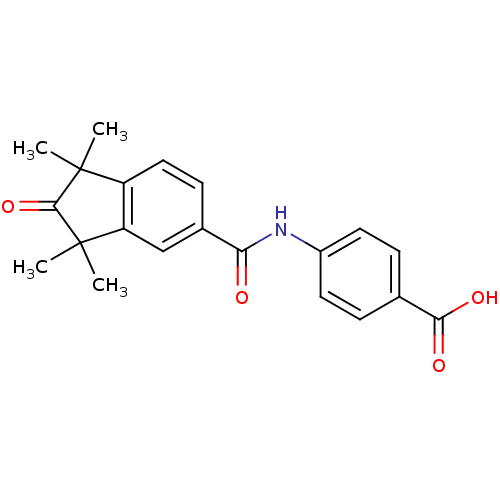 (BMS753 | US9963439, BMS753) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM393289 (US9963439, BMS961) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26A1 (Homo sapiens (Human)) | BDBM36810 (BMS753 | US9963439, BMS753) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 4.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Eighteen compounds were tested as potential inhibitors of CYP26A1 and CYP26B1. The formation of 9-cis-4-OH-RA metabolite was monitored and the percen... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26A1 (Homo sapiens (Human)) | BDBM393294 (US9963439, Compound E) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Eighteen compounds were tested as potential inhibitors of CYP26A1 and CYP26B1. The formation of 9-cis-4-OH-RA metabolite was monitored and the percen... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||