

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087205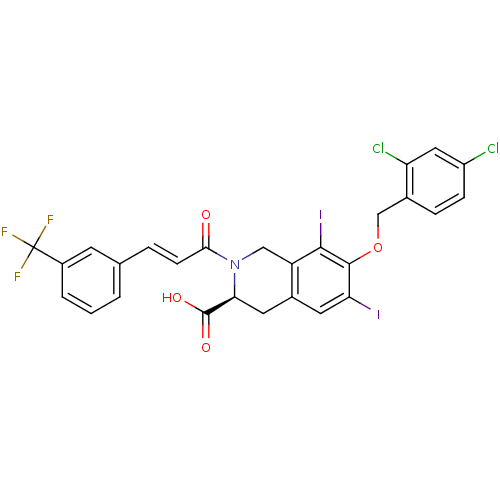 ((S)-7-(2,4-Dichloro-benzyloxy)-6,8-diiodo-2-[(E)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087187 ((S)-2-[(E)-3-(2-Chloro-phenyl)-acryloyl]-7-(2,4-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087179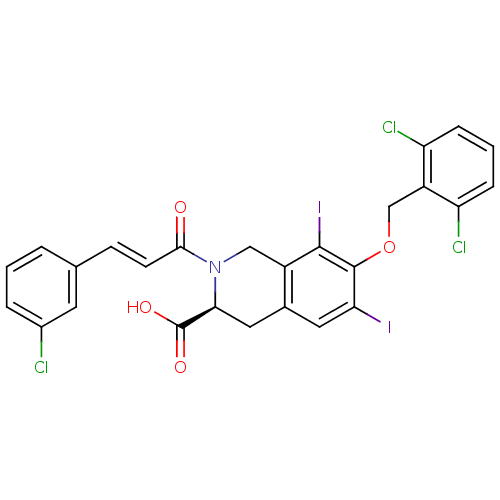 ((S)-2-[(E)-3-(3-Chloro-phenyl)-acryloyl]-7-(2,6-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087234 ((S)-7-(2,4-Dichloro-benzyloxy)-2-[(E)-3-(3,5-dichl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087231 ((S)-2-[(E)-3-(4-Bromo-phenyl)-acryloyl]-7-(2,4-dic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087191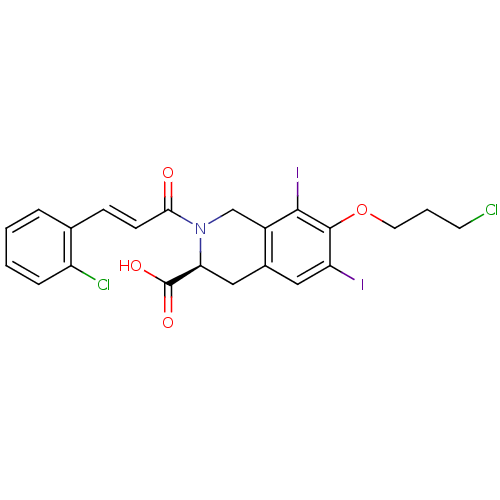 ((S)-2-[(E)-3-(2-Chloro-phenyl)-acryloyl]-7-(3-chlo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087225 ((S)-7-(2,4-Dichloro-benzyloxy)-6,8-diiodo-2-((E)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087227 ((S)-6,8-Diiodo-7-(4-trifluoromethyl-benzyloxy)-2-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087189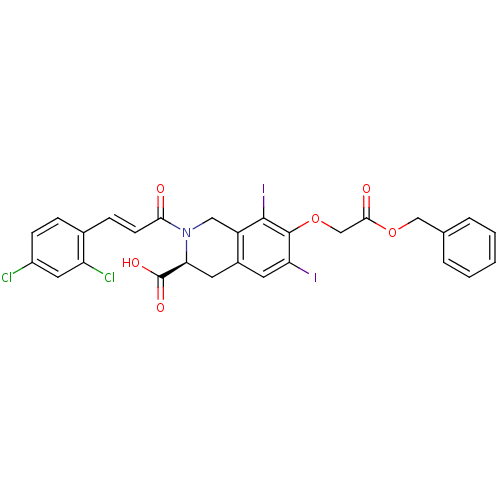 ((S)-7-Benzyloxycarbonylmethoxy-2-[(E)-3-(2,4-dichl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087199 ((S)-2-[(E)-3-(3-Fluoro-phenyl)-acryloyl]-6,8-diiod...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087188 ((S)-2-(2-Chloro-pyridine-3-carbonyl)-6,8-diiodo-7-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087173 ((S)-6,8-Diiodo-2-((E)-3-p-tolyl-acryloyl)-7-(3-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087196 ((S)-7-(2,4-Dichloro-benzyloxy)-2-[(E)-3-(3,4-dichl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087213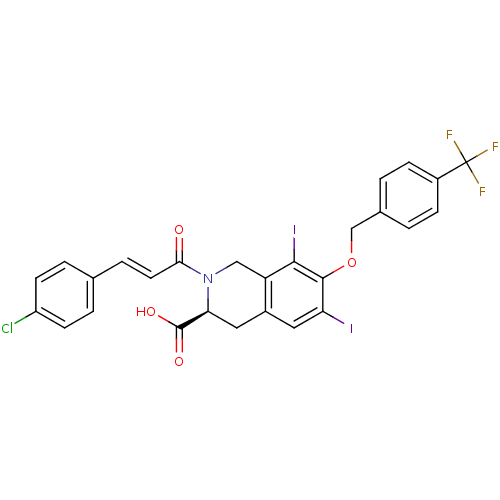 ((S)-2-[(E)-3-(4-Chloro-phenyl)-acryloyl]-6,8-diiod...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087183 ((S)-2-((E)-3-2,4-Dioxa-bicyclo[3.3.1]nona-1(8),5(9...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087224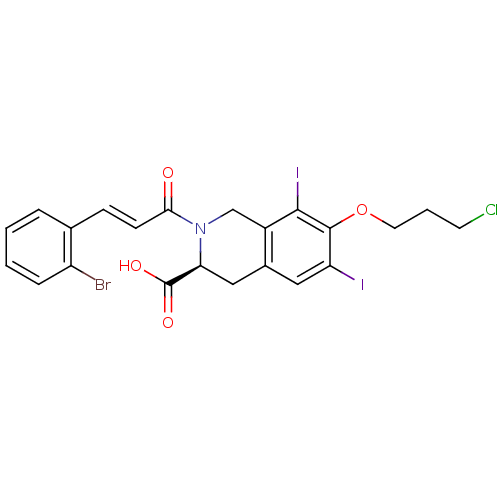 ((S)-2-[(E)-3-(2-Bromo-phenyl)-acryloyl]-7-(3-chlor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087206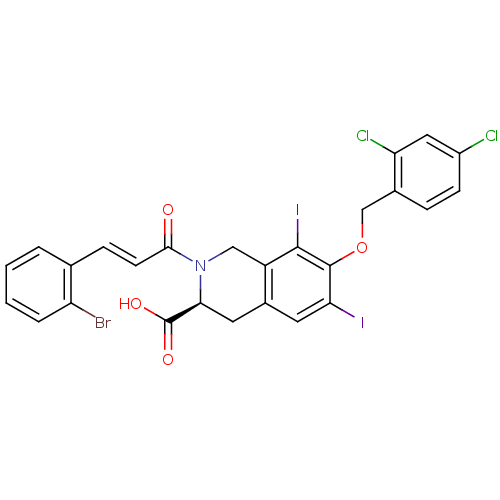 ((S)-2-[(E)-3-(2-Bromo-phenyl)-acryloyl]-7-(2,4-dic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087204 ((3S)-7-[(2,6-dichlorophenyl)methoxy]-6,8-diiodo-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087197 ((S)-2-[(E)-3-(2-Fluoro-phenyl)-acryloyl]-6,8-diiod...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087203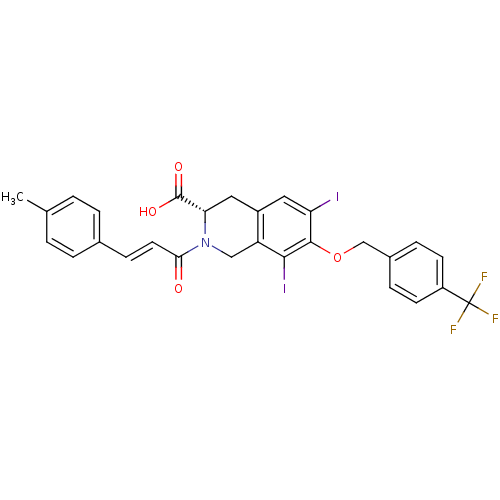 ((S)-6,8-Diiodo-2-((E)-3-p-tolyl-acryloyl)-7-(4-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087208 ((S)-2-[(E)-3-(4-Chloro-phenyl)-acryloyl]-7-(2,4-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087223 ((S)-2-[(E)-3-(3-Chloro-phenyl)-acryloyl]-7-(2,4-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087235 ((S)-7-(2,4-Dichloro-benzyloxy)-2-((Z)-2-fluoro-3-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087198 ((S)-6,8-Diiodo-7-(3-trifluoromethyl-benzyloxy)-2-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087186 ((S)-7-(2,4-Dichloro-benzyloxy)-6,8-diiodo-2-((E)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087181 ((S)-7-(2,4-Dichloro-benzyloxy)-2-[(E)-3-(3-fluoro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087202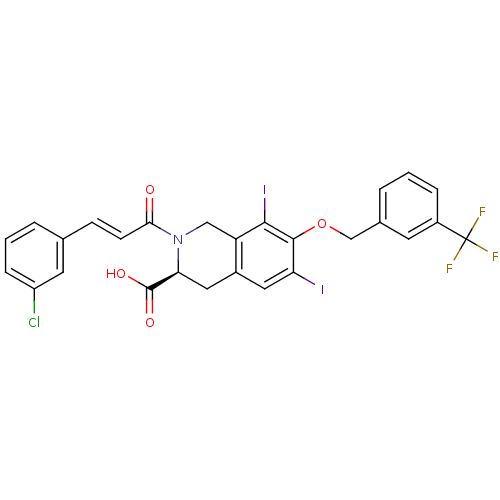 ((S)-2-[(E)-3-(3-Chloro-phenyl)-acryloyl]-6,8-diiod...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087200 ((S)-2-[(E)-3-(3-Bromo-phenyl)-acryloyl]-7-(2,4-dic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087201 ((S)-7-(2-Fluoro-benzyloxy)-6,8-diiodo-2-[(E)-3-(3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087190 ((S)-2-[(E)-3-(3-Fluoro-phenyl)-acryloyl]-6,8-diiod...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087195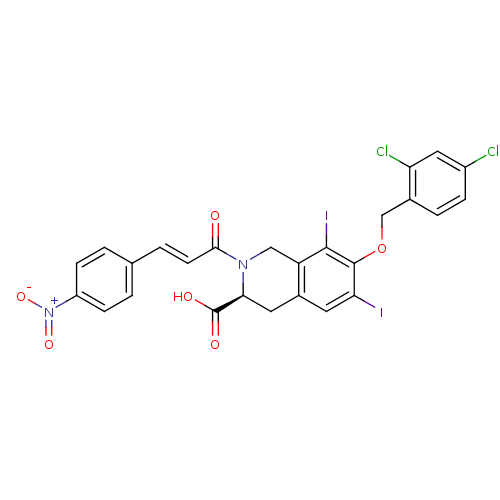 ((S)-7-(2,4-Dichloro-benzyloxy)-6,8-diiodo-2-[(E)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087228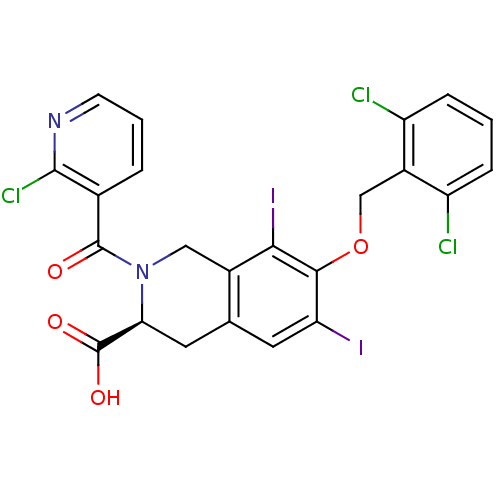 ((S)-2-(2-Chloro-pyridine-3-carbonyl)-7-(2,6-dichlo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087232 ((S)-7-(2,6-Dichloro-benzyloxy)-2-[(E)-3-(3-fluoro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087226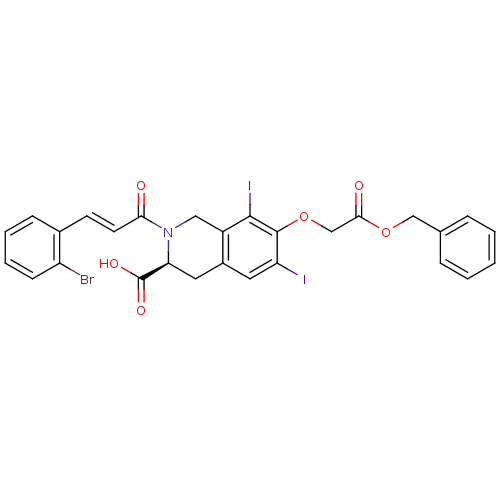 ((S)-7-Benzyloxycarbonylmethoxy-2-[(E)-3-(2-bromo-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087193 ((S)-6,8-Diiodo-2-(3-phenyl-propionyl)-7-(3-trifluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087230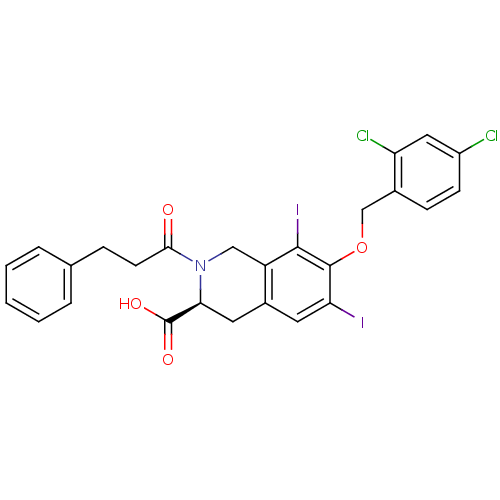 ((S)-7-(2,4-Dichloro-benzyloxy)-6,8-diiodo-2-(3-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087176 ((S)-6,8-Diiodo-2-[(E)-3-(4-nitro-phenyl)-acryloyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087209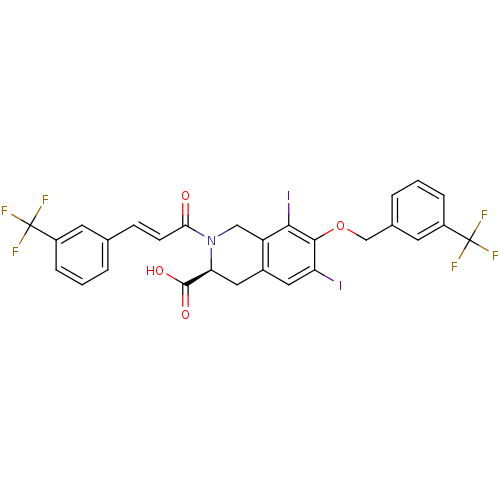 ((S)-6,8-Diiodo-7-(3-trifluoromethyl-benzyloxy)-2-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087233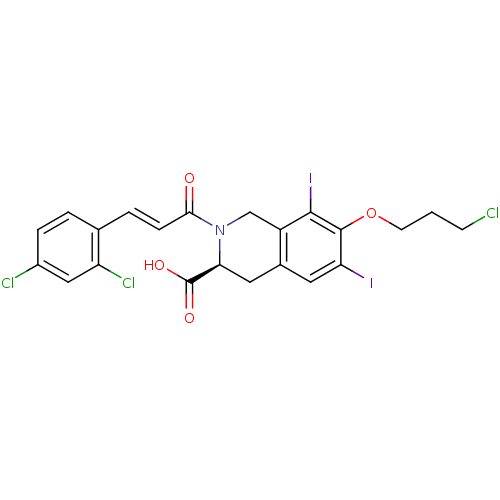 ((S)-7-(3-Chloro-propoxy)-2-[(E)-3-(2,4-dichloro-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087174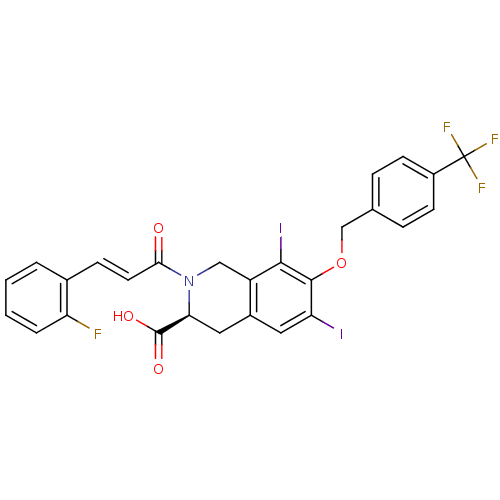 ((S)-2-[(E)-3-(2-Fluoro-phenyl)-acryloyl]-6,8-diiod...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087222 ((S)-7-(2,6-Dichloro-benzyloxy)-2-[(E)-3-(3,4-dichl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087215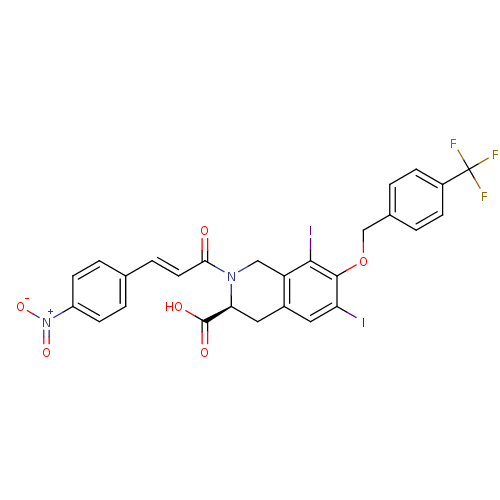 ((S)-6,8-Diiodo-2-[(E)-3-(4-nitro-phenyl)-acryloyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087184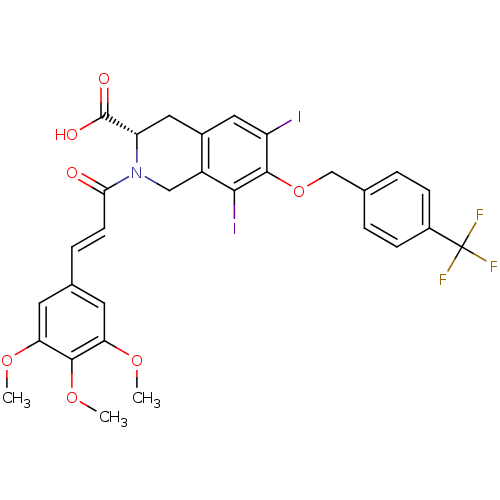 ((S)-6,8-Diiodo-7-(4-trifluoromethyl-benzyloxy)-2-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087185 ((S)-2-((E)-3-2,4-Dioxa-bicyclo[3.3.1]nona-1(8),5(9...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087210 ((S)-6,8-Diiodo-7-(3-methoxy-benzyloxy)-2-[(E)-3-(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087211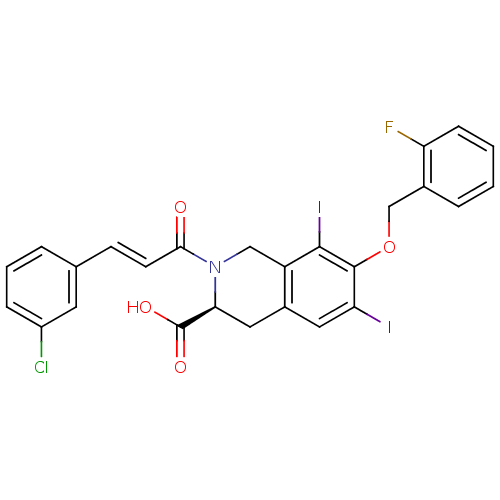 ((S)-2-[(E)-3-(3-Chloro-phenyl)-acryloyl]-7-(2-fluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087194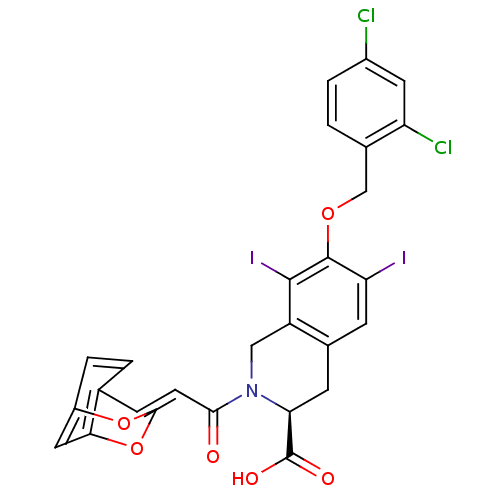 ((S)-7-(2,4-Dichloro-benzyloxy)-2-((E)-3-2,4-dioxa-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087216 ((S)-6,8-Diiodo-7-(3-methoxy-benzyloxy)-2-((E)-3-p-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087221 ((S)-2-((E)-3-2,4-Dioxa-bicyclo[3.3.1]nona-1(8),5(9...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087236 ((S)-2-[(E)-3-(4-Bromo-phenyl)-acryloyl]-7-(3-chlor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087214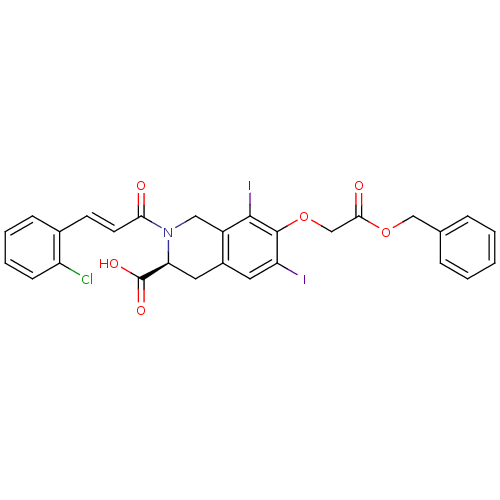 ((S)-7-Benzyloxycarbonylmethoxy-2-[(E)-3-(2-chloro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087178 ((S)-2-[(E)-3-(3-Bromo-phenyl)-acryloyl]-7-(3-chlor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087175 ((S)-2-[(E)-3-(4-Chloro-phenyl)-acryloyl]-6,8-diiod...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087219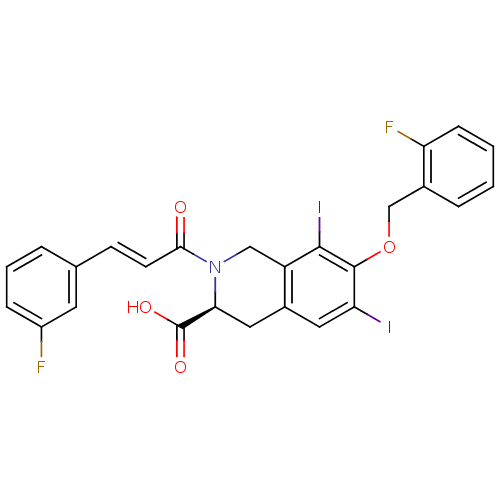 ((S)-7-(2-Fluoro-benzyloxy)-2-[(E)-3-(3-fluoro-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087220 ((S)-2-[(E)-3-(3,4-Dichloro-phenyl)-acryloyl]-6,8-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087180 ((S)-2-[(E)-3-(3,4-Dichloro-phenyl)-acryloyl]-7-(2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087212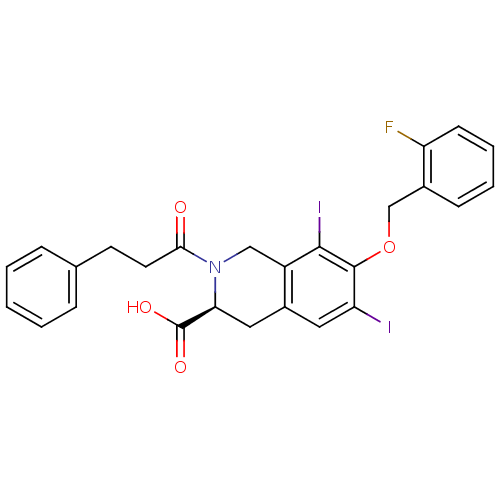 ((S)-7-(2-Fluoro-benzyloxy)-6,8-diiodo-2-(3-phenyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087192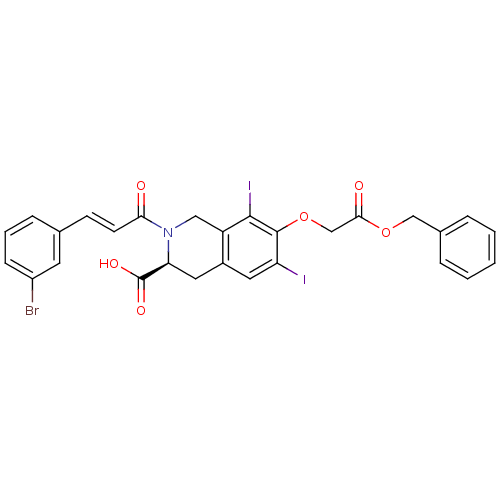 ((S)-7-Benzyloxycarbonylmethoxy-2-[(E)-3-(3-bromo-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087207 ((S)-7-Benzyloxycarbonylmethoxy-2-[(E)-3-(4-bromo-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087229 ((S)-7-Benzyloxycarbonylmethoxy-2-((Z)-2-fluoro-3-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087172 ((S)-2-(2-Chloro-pyridine-3-carbonyl)-7-(2-fluoro-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087218 ((S)-2-(2-Chloro-pyridine-3-carbonyl)-7-(2,4-dichlo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087217 ((S)-7-(3-Chloro-propoxy)-2-((Z)-2-fluoro-3-phenyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087182 (2-Cyclopentanecarbonyl-7-(2,6-dichloro-benzyloxy)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087177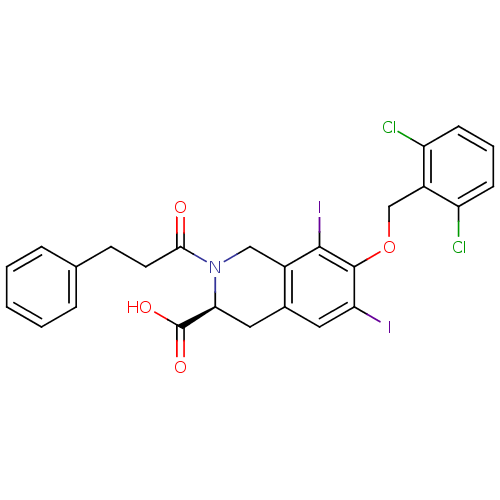 ((S)-7-(2,6-Dichloro-benzyloxy)-6,8-diiodo-2-(3-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Company Curated by ChEMBL | Assay Description Compound was measured for its inhibitory activity against Cell division cycle 25B | Bioorg Med Chem Lett 10: 649-52 (2000) BindingDB Entry DOI: 10.7270/Q2KS6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||