 Found 10 hits of Enzyme Inhibition Constant Data
Found 10 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM22875
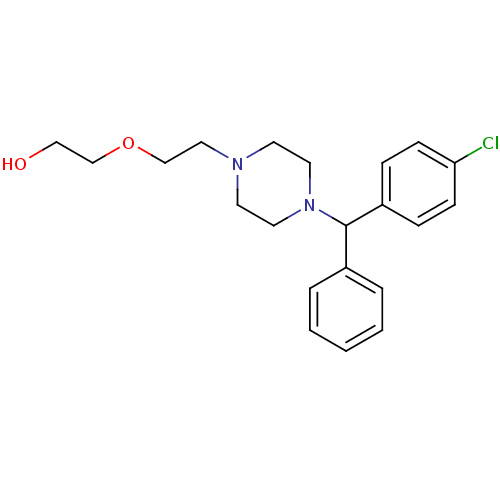
(2-(2-{4-[(4-chlorophenyl)(phenyl)methyl]piperazin-...)Show InChI InChI=1S/C21H27ClN2O2/c22-20-8-6-19(7-9-20)21(18-4-2-1-3-5-18)24-12-10-23(11-13-24)14-16-26-17-15-25/h1-9,21,25H,10-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB S.A.
Curated by PDSP Ki Database
| |
Mol Pharmacol 61: 391-9 (2002)
Article DOI: 10.1124/mol.61.2.391
BindingDB Entry DOI: 10.7270/Q2D50KJ8 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM22875

(2-(2-{4-[(4-chlorophenyl)(phenyl)methyl]piperazin-...)Show InChI InChI=1S/C21H27ClN2O2/c22-20-8-6-19(7-9-20)21(18-4-2-1-3-5-18)24-12-10-23(11-13-24)14-16-26-17-15-25/h1-9,21,25H,10-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB S.A.
Curated by PDSP Ki Database
| |
Mol Pharmacol 61: 391-9 (2002)
Article DOI: 10.1124/mol.61.2.391
BindingDB Entry DOI: 10.7270/Q2D50KJ8 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM22879
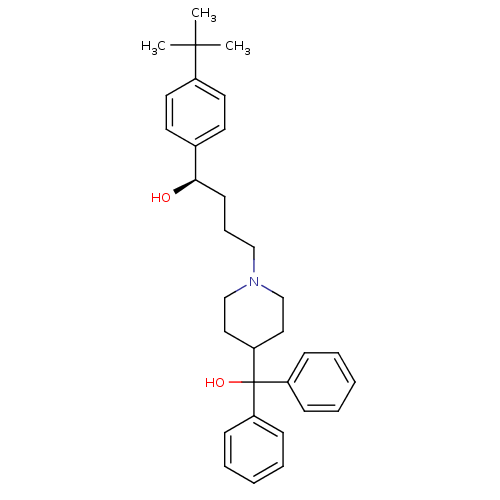
((1R)-1-(4-tert-butylphenyl)-4-[4-(hydroxydiphenylm...)Show SMILES CC(C)(C)c1ccc(cc1)[C@H](O)CCCN1CCC(CC1)C(O)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C32H41NO2/c1-31(2,3)26-18-16-25(17-19-26)30(34)15-10-22-33-23-20-29(21-24-33)32(35,27-11-6-4-7-12-27)28-13-8-5-9-14-28/h4-9,11-14,16-19,29-30,34-35H,10,15,20-24H2,1-3H3/t30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB S.A.
Curated by PDSP Ki Database
| |
Mol Pharmacol 61: 391-9 (2002)
Article DOI: 10.1124/mol.61.2.391
BindingDB Entry DOI: 10.7270/Q2D50KJ8 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM81466
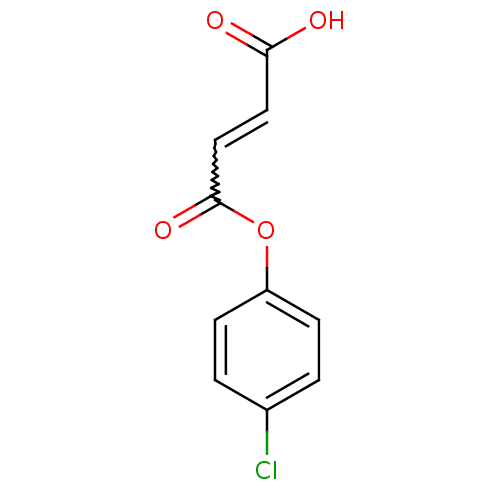
(CAS_23095-76-3 | NSC_6445134 | l-Chlorpheniramine)Show InChI InChI=1S/C10H7ClO4/c11-7-1-3-8(4-2-7)15-10(14)6-5-9(12)13/h1-6H,(H,12,13) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB S.A.
Curated by PDSP Ki Database
| |
Mol Pharmacol 61: 391-9 (2002)
Article DOI: 10.1124/mol.61.2.391
BindingDB Entry DOI: 10.7270/Q2D50KJ8 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM22890

(2-(2-{4-[(4-chlorophenyl)(phenyl)methyl]piperazin-...)Show SMILES OC(=O)COCCN1CCN(CC1)C(c1ccccc1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H25ClN2O3/c22-19-8-6-18(7-9-19)21(17-4-2-1-3-5-17)24-12-10-23(11-13-24)14-15-27-16-20(25)26/h1-9,21H,10-16H2,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB S.A.
Curated by PDSP Ki Database
| |
Mol Pharmacol 61: 391-9 (2002)
Article DOI: 10.1124/mol.61.2.391
BindingDB Entry DOI: 10.7270/Q2D50KJ8 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM85849

(ucb 29992, (R))Show SMILES COC(=O)COCCN1CCN(CC1)[C@H](c1ccccc1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C22H27ClN2O3/c1-27-21(26)17-28-16-15-24-11-13-25(14-12-24)22(18-5-3-2-4-6-18)19-7-9-20(23)10-8-19/h2-10,22H,11-17H2,1H3/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB S.A.
Curated by PDSP Ki Database
| |
Mol Pharmacol 61: 391-9 (2002)
Article DOI: 10.1124/mol.61.2.391
BindingDB Entry DOI: 10.7270/Q2D50KJ8 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM22874
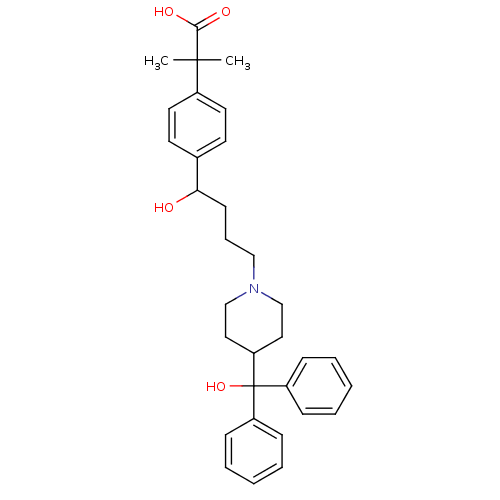
(2-(4-{1-hydroxy-4-[4-(hydroxydiphenylmethyl)piperi...)Show SMILES CC(C)(C(O)=O)c1ccc(cc1)C(O)CCCN1CCC(CC1)C(O)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C32H39NO4/c1-31(2,30(35)36)25-17-15-24(16-18-25)29(34)14-9-21-33-22-19-28(20-23-33)32(37,26-10-5-3-6-11-26)27-12-7-4-8-13-27/h3-8,10-13,15-18,28-29,34,37H,9,14,19-23H2,1-2H3,(H,35,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB S.A.
Curated by PDSP Ki Database
| |
Mol Pharmacol 61: 391-9 (2002)
Article DOI: 10.1124/mol.61.2.391
BindingDB Entry DOI: 10.7270/Q2D50KJ8 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM85850

(ucb 29993, (S))Show SMILES COC(=O)COCCN1CCN(CC1)[C@@H](c1ccccc1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C22H27ClN2O3/c1-27-21(26)17-28-16-15-24-11-13-25(14-12-24)22(18-5-3-2-4-6-18)19-7-9-20(23)10-8-19/h2-10,22H,11-17H2,1H3/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 63.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB S.A.
Curated by PDSP Ki Database
| |
Mol Pharmacol 61: 391-9 (2002)
Article DOI: 10.1124/mol.61.2.391
BindingDB Entry DOI: 10.7270/Q2D50KJ8 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM85029
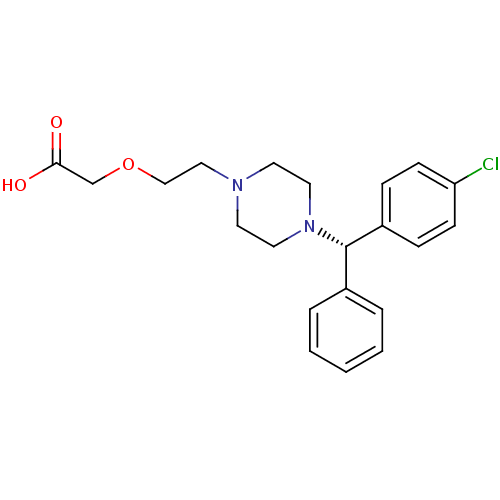
(Cetirizine (+) isomer)Show SMILES OC(=O)COCCN1CCN(CC1)[C@@H](c1ccccc1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C21H25ClN2O3/c22-19-8-6-18(7-9-19)21(17-4-2-1-3-5-17)24-12-10-23(11-13-24)14-15-27-16-20(25)26/h1-9,21H,10-16H2,(H,25,26)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 79.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB S.A.
Curated by PDSP Ki Database
| |
Mol Pharmacol 61: 391-9 (2002)
Article DOI: 10.1124/mol.61.2.391
BindingDB Entry DOI: 10.7270/Q2D50KJ8 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM7966

(2-(1H-imidazol-4-yl)ethan-1-amine | CHEMBL544208 |...)Show InChI InChI=1S/C5H9N3/c6-2-1-5-3-7-4-8-5/h3-4H,1-2,6H2,(H,7,8) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB S.A.
Curated by PDSP Ki Database
| |
Mol Pharmacol 61: 391-9 (2002)
Article DOI: 10.1124/mol.61.2.391
BindingDB Entry DOI: 10.7270/Q2D50KJ8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data