

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Oryctolagus cuniculus) | BDBM50079240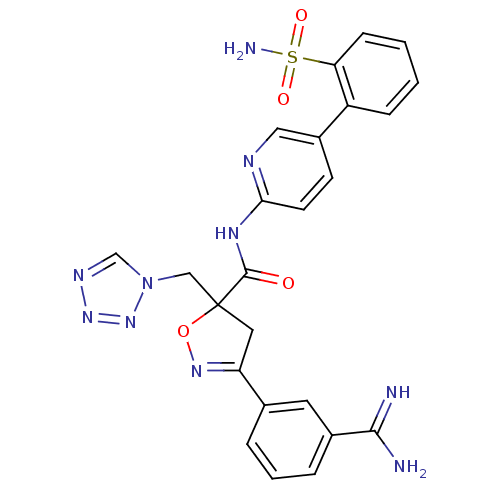 (3-(3-Carbamimidoyl-phenyl)-5-tetrazol-1-ylmethyl-4...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against Rabbit factor Xa was determined | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50079240 (3-(3-Carbamimidoyl-phenyl)-5-tetrazol-1-ylmethyl-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against factor Xa using human purified enzyme. | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50079240 (3-(3-Carbamimidoyl-phenyl)-5-tetrazol-1-ylmethyl-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against trypsin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50125240 (3-(3-Amino-4-chloro-phenyl)-5-[(thiophene-3-sulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against factor Xa using human purified enzyme. | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50125242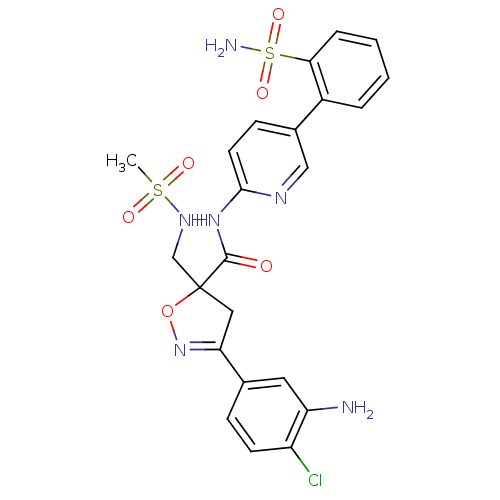 (3-(3-Amino-4-chloro-phenyl)-5-(methanesulfonylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50125245 (3-(3-Amino-4-chloro-phenyl)-5-(benzenesulfonylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against factor Xa using human purified enzyme. | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50125242 (3-(3-Amino-4-chloro-phenyl)-5-(methanesulfonylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50125241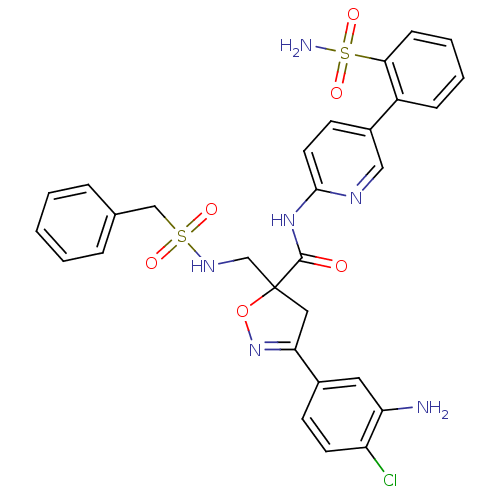 (3-(3-Amino-4-chloro-phenyl)-5-(phenylmethanesulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against factor Xa using human purified enzyme. | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Oryctolagus cuniculus) | BDBM50125242 (3-(3-Amino-4-chloro-phenyl)-5-(methanesulfonylamin...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against Rabbit factor Xa was determined | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50125243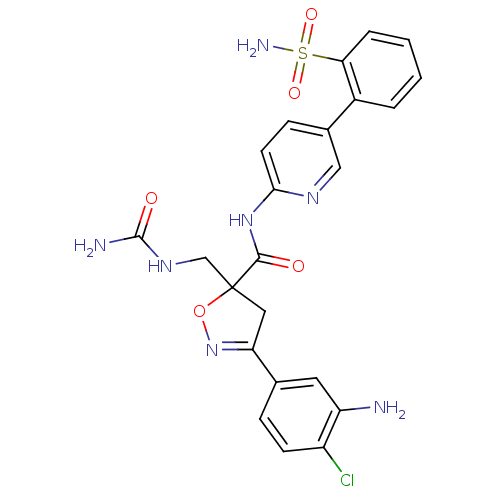 (3-(3-Amino-4-chloro-phenyl)-5-ureidomethyl-4,5-dih...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against Rabbit factor Xa was determined | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50125243 (3-(3-Amino-4-chloro-phenyl)-5-ureidomethyl-4,5-dih...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against factor Xa using human purified enzyme. | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50125250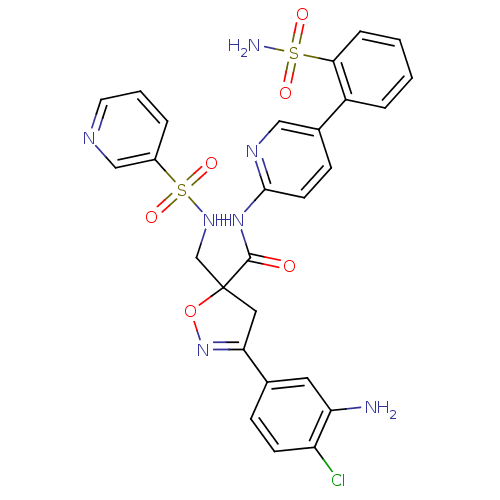 (3-(3-Amino-4-chloro-phenyl)-5-[(pyridine-3-sulfony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against factor Xa using human purified enzyme. | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50125236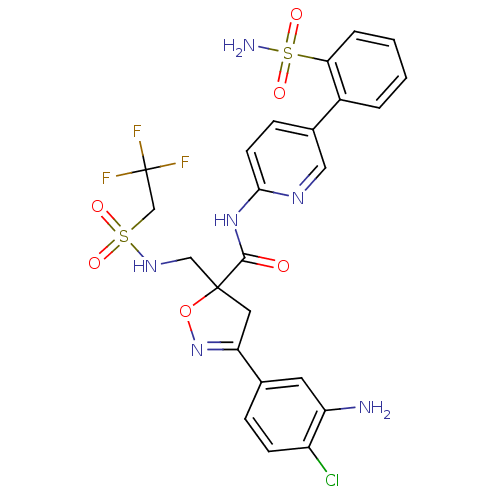 (3-(3-Amino-4-chloro-phenyl)-5-[(2,2,2-trifluoro-et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against factor Xa using human purified enzyme. | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50125246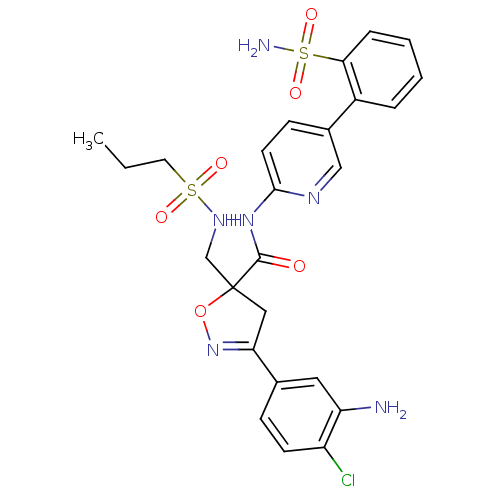 (3-(3-Amino-4-chloro-phenyl)-5-[(propane-1-sulfonyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against factor Xa using human purified enzyme. | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50125251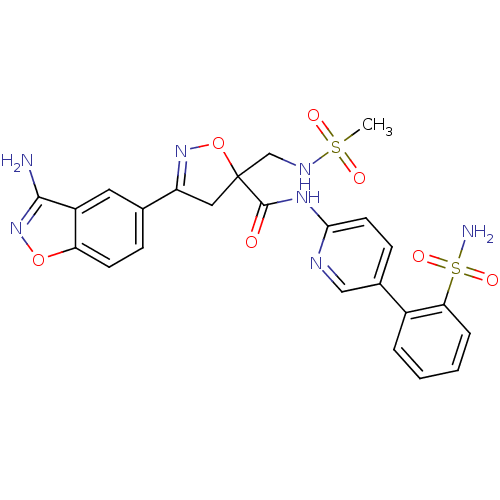 (3-(3-Amino-benzo[d]isoxazol-5-yl)-5-(methanesulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against factor Xa using human purified enzyme. | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Oryctolagus cuniculus) | BDBM50125243 (3-(3-Amino-4-chloro-phenyl)-5-ureidomethyl-4,5-dih...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against Rabbit factor Xa was determined | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50125254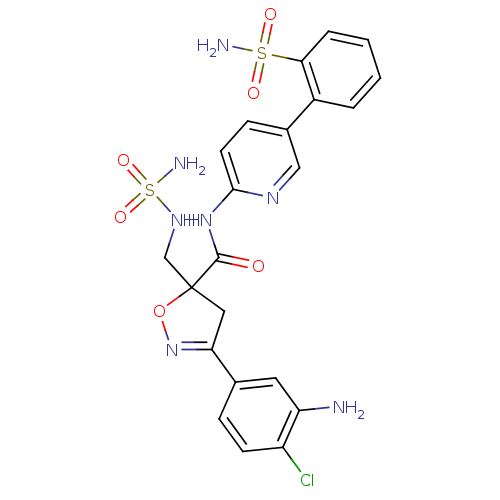 (3-(3-Amino-4-chloro-phenyl)-5-(aminosulfonylamino-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against factor Xa using human purified enzyme. | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50125237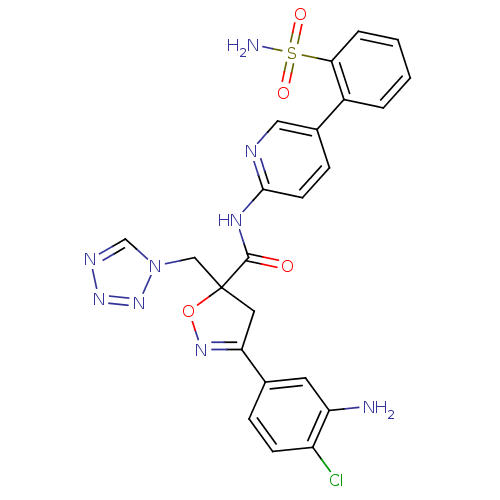 (3-(3-Amino-4-chloro-phenyl)-5-tetrazol-1-ylmethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Binding affinity towards alpha4-beta2 nicotinic receptor was determined in rat brain membrane using [3H]cytisine as radioligand | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50079247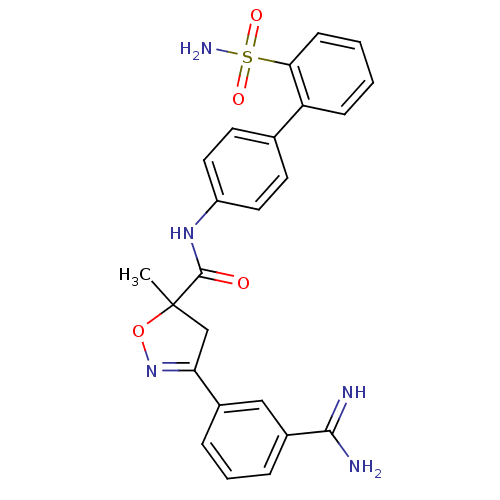 (3-(3-Carbamimidoyl-phenyl)-5-methyl-4,5-dihydro-is...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against factor Xa using human purified enzyme. | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50125238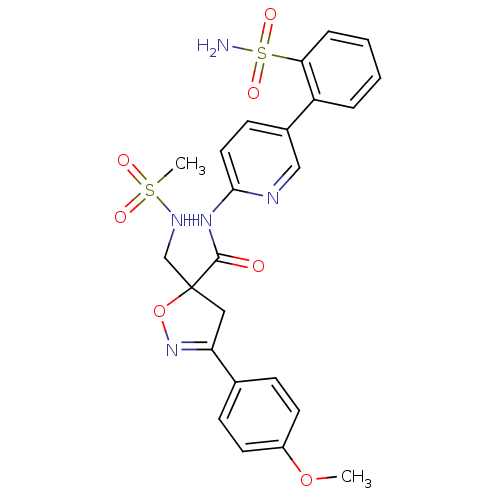 (5-(Methanesulfonylamino-methyl)-3-(4-methoxy-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against factor Xa using human purified enzyme. | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50125237 (3-(3-Amino-4-chloro-phenyl)-5-tetrazol-1-ylmethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Binding affinity towards alpha4-beta2 nicotinic receptor was determined in rat brain membrane using [3H]cytisine as radioligand | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50125248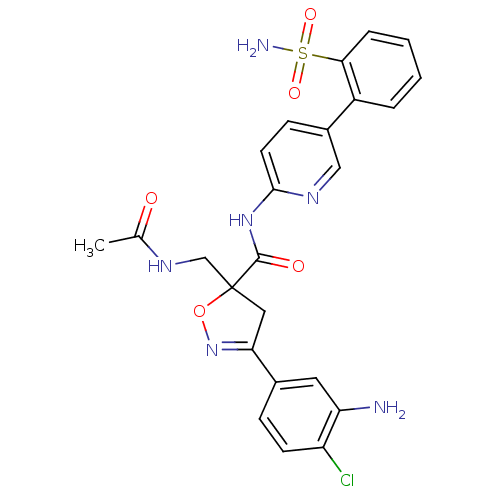 (5-(Acetylamino-methyl)-3-(3-amino-4-chloro-phenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against factor Xa using human purified enzyme. | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50125239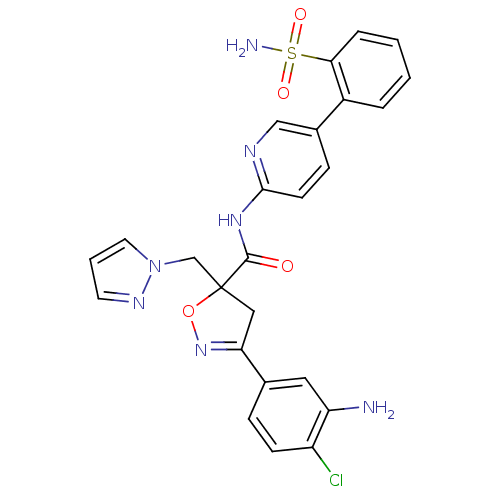 (3-(3-Amino-4-chloro-phenyl)-5-pyrazol-1-ylmethyl-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against factor Xa using human purified enzyme. | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50125239 (3-(3-Amino-4-chloro-phenyl)-5-pyrazol-1-ylmethyl-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50125249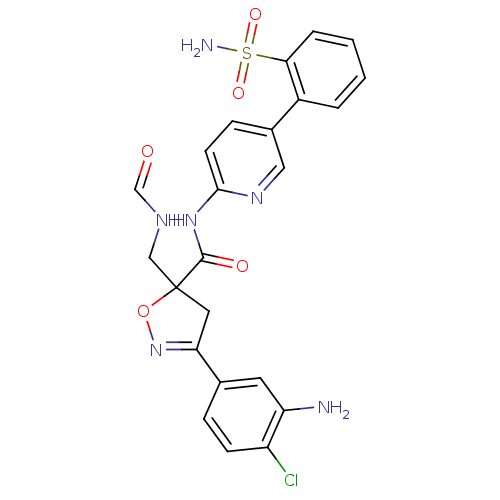 (3-(3-Amino-4-chloro-phenyl)-5-formylaminomethyl-4,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against factor Xa using human purified enzyme. | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50079240 (3-(3-Carbamimidoyl-phenyl)-5-tetrazol-1-ylmethyl-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against trypsin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50125253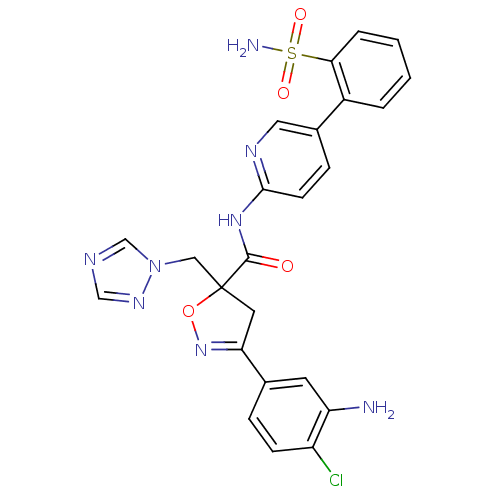 (3-(3-Amino-4-chloro-phenyl)-5-[1,2,4]triazol-1-ylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against factor Xa using human purified enzyme. | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50125252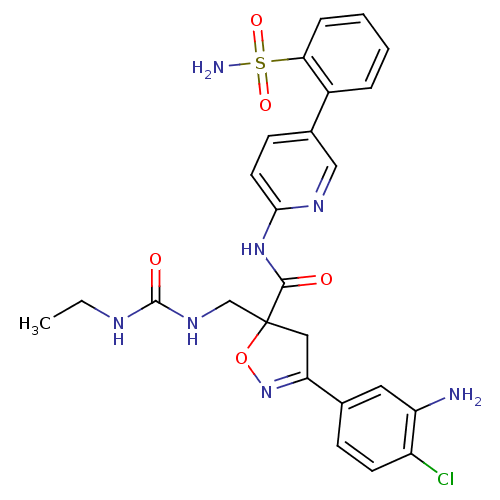 (3-(3-Amino-4-chloro-phenyl)-5-[(3-ethyl-ureido)-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against factor Xa using human purified enzyme. | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50125243 (3-(3-Amino-4-chloro-phenyl)-5-ureidomethyl-4,5-dih...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against factor Xa using human purified enzyme. | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50125244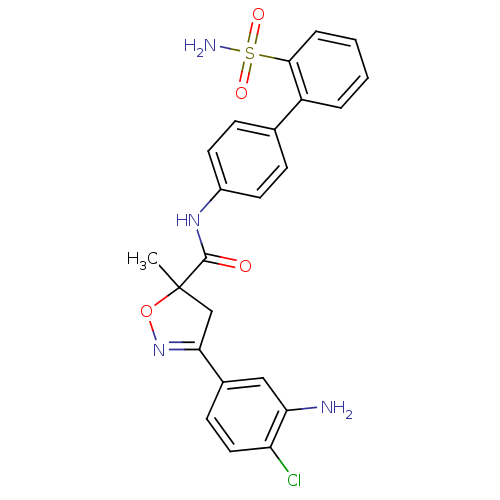 (3-(3-Amino-4-chloro-phenyl)-5-methyl-4,5-dihydro-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against factor Xa using human purified enzyme. | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50125242 (3-(3-Amino-4-chloro-phenyl)-5-(methanesulfonylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against Rabbit factor Xa was determined | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50125237 (3-(3-Amino-4-chloro-phenyl)-5-tetrazol-1-ylmethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against factor Xa using human purified enzyme. | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50125242 (3-(3-Amino-4-chloro-phenyl)-5-(methanesulfonylamin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50125243 (3-(3-Amino-4-chloro-phenyl)-5-ureidomethyl-4,5-dih...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against factor Xa using human purified enzyme. | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50079240 (3-(3-Carbamimidoyl-phenyl)-5-tetrazol-1-ylmethyl-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50125239 (3-(3-Amino-4-chloro-phenyl)-5-pyrazol-1-ylmethyl-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against factor Xa using human purified enzyme. | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50125242 (3-(3-Amino-4-chloro-phenyl)-5-(methanesulfonylamin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50125239 (3-(3-Amino-4-chloro-phenyl)-5-pyrazol-1-ylmethyl-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50125239 (3-(3-Amino-4-chloro-phenyl)-5-pyrazol-1-ylmethyl-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against trypsin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50125243 (3-(3-Amino-4-chloro-phenyl)-5-ureidomethyl-4,5-dih...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against trypsin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50125237 (3-(3-Amino-4-chloro-phenyl)-5-tetrazol-1-ylmethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against factor Xa using human purified enzyme. | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50125248 (5-(Acetylamino-methyl)-3-(3-amino-4-chloro-phenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against trypsin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50125237 (3-(3-Amino-4-chloro-phenyl)-5-tetrazol-1-ylmethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against trypsin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50125243 (3-(3-Amino-4-chloro-phenyl)-5-ureidomethyl-4,5-dih...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Binding affinity towards alpha4-beta2 nicotinic receptor was determined in rat brain membrane using [3H]cytisine as radioligand | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50125242 (3-(3-Amino-4-chloro-phenyl)-5-(methanesulfonylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against trypsin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50125241 (3-(3-Amino-4-chloro-phenyl)-5-(phenylmethanesulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against trypsin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50125252 (3-(3-Amino-4-chloro-phenyl)-5-[(3-ethyl-ureido)-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against trypsin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50125238 (5-(Methanesulfonylamino-methyl)-3-(4-methoxy-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against trypsin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50125253 (3-(3-Amino-4-chloro-phenyl)-5-[1,2,4]triazol-1-ylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against trypsin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50125251 (3-(3-Amino-benzo[d]isoxazol-5-yl)-5-(methanesulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against trypsin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50125251 (3-(3-Amino-benzo[d]isoxazol-5-yl)-5-(methanesulfon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50125254 (3-(3-Amino-4-chloro-phenyl)-5-(aminosulfonylamino-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50125243 (3-(3-Amino-4-chloro-phenyl)-5-ureidomethyl-4,5-dih...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Potency on ETA receptor assessed by inhibition of the contraction induced by ET-1 in rat aortic rings | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50125241 (3-(3-Amino-4-chloro-phenyl)-5-(phenylmethanesulfon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50125252 (3-(3-Amino-4-chloro-phenyl)-5-[(3-ethyl-ureido)-me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50125238 (5-(Methanesulfonylamino-methyl)-3-(4-methoxy-pheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50125236 (3-(3-Amino-4-chloro-phenyl)-5-[(2,2,2-trifluoro-et...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50125250 (3-(3-Amino-4-chloro-phenyl)-5-[(pyridine-3-sulfony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against trypsin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50125245 (3-(3-Amino-4-chloro-phenyl)-5-(benzenesulfonylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against trypsin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50125242 (3-(3-Amino-4-chloro-phenyl)-5-(methanesulfonylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against trypsin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50125240 (3-(3-Amino-4-chloro-phenyl)-5-[(thiophene-3-sulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against trypsin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50125236 (3-(3-Amino-4-chloro-phenyl)-5-[(2,2,2-trifluoro-et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against trypsin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50125254 (3-(3-Amino-4-chloro-phenyl)-5-(aminosulfonylamino-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against trypsin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50125242 (3-(3-Amino-4-chloro-phenyl)-5-(methanesulfonylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against trypsin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50125246 (3-(3-Amino-4-chloro-phenyl)-5-[(propane-1-sulfonyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against trypsin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50125249 (3-(3-Amino-4-chloro-phenyl)-5-formylaminomethyl-4,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against trypsin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50125243 (3-(3-Amino-4-chloro-phenyl)-5-ureidomethyl-4,5-dih...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against factor Xa using human purified enzyme. | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50125249 (3-(3-Amino-4-chloro-phenyl)-5-formylaminomethyl-4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50125250 (3-(3-Amino-4-chloro-phenyl)-5-[(pyridine-3-sulfony...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50125246 (3-(3-Amino-4-chloro-phenyl)-5-[(propane-1-sulfonyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50125237 (3-(3-Amino-4-chloro-phenyl)-5-tetrazol-1-ylmethyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50125248 (5-(Acetylamino-methyl)-3-(3-amino-4-chloro-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50125243 (3-(3-Amino-4-chloro-phenyl)-5-ureidomethyl-4,5-dih...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50125239 (3-(3-Amino-4-chloro-phenyl)-5-pyrazol-1-ylmethyl-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50125253 (3-(3-Amino-4-chloro-phenyl)-5-[1,2,4]triazol-1-ylm...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50125239 (3-(3-Amino-4-chloro-phenyl)-5-pyrazol-1-ylmethyl-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against trypsin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50125245 (3-(3-Amino-4-chloro-phenyl)-5-(benzenesulfonylamin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50125240 (3-(3-Amino-4-chloro-phenyl)-5-[(thiophene-3-sulfon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50125237 (3-(3-Amino-4-chloro-phenyl)-5-tetrazol-1-ylmethyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50125242 (3-(3-Amino-4-chloro-phenyl)-5-(methanesulfonylamin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50125237 (3-(3-Amino-4-chloro-phenyl)-5-tetrazol-1-ylmethyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||