

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50130610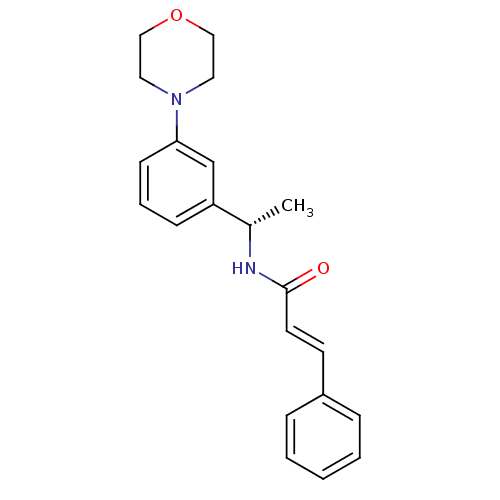 ((E)-N-[(S)-1-(3-Morpholin-4-yl-phenyl)-ethyl]-3-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human Cytochrome P450 2C9 | J Med Chem 46: 3778-81 (2003) Article DOI: 10.1021/jm034111v BindingDB Entry DOI: 10.7270/Q2RJ4K66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50130610 ((E)-N-[(S)-1-(3-Morpholin-4-yl-phenyl)-ethyl]-3-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human Cytochrome P450 19A1 | J Med Chem 46: 3778-81 (2003) Article DOI: 10.1021/jm034111v BindingDB Entry DOI: 10.7270/Q2RJ4K66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50370258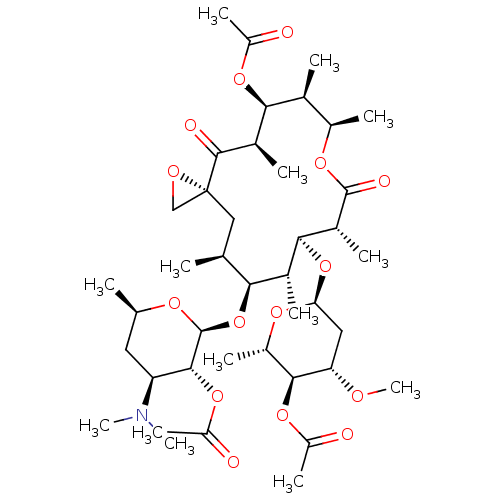 (TROLEANDOMYCIN | Triacetyloleandomycin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | DrugBank Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human Cytochrome P450 3A4 with BFC [7-benzyloxy-4-trifluoromethylcoumarin] after 45 minutes | J Med Chem 46: 3778-81 (2003) Article DOI: 10.1021/jm034111v BindingDB Entry DOI: 10.7270/Q2RJ4K66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50131898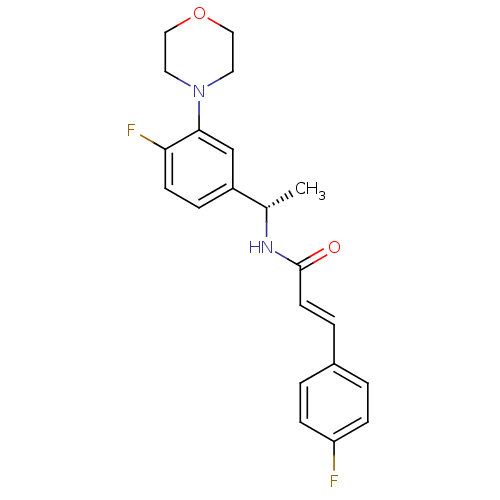 ((E)-N-[1-((S)-4-Fluoro-3-morpholin-4-yl-phenyl)-et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human Cytochrome P450 3A4 with BFC [7-benzyloxy-4-trifluoromethylcoumarin] after 5 minutes | J Med Chem 46: 3778-81 (2003) Article DOI: 10.1021/jm034111v BindingDB Entry DOI: 10.7270/Q2RJ4K66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50131898 ((E)-N-[1-((S)-4-Fluoro-3-morpholin-4-yl-phenyl)-et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human Cytochrome P450 3A4 using BFC [7-benzyloxy-4-trifluoromethylcoumarin] after 30 minutes | J Med Chem 46: 3778-81 (2003) Article DOI: 10.1021/jm034111v BindingDB Entry DOI: 10.7270/Q2RJ4K66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50131898 ((E)-N-[1-((S)-4-Fluoro-3-morpholin-4-yl-phenyl)-et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human Cytochrome P450 3A4 with BFC [7-benzyloxy-4-trifluoromethylcoumarin] after 45 minutes | J Med Chem 46: 3778-81 (2003) Article DOI: 10.1021/jm034111v BindingDB Entry DOI: 10.7270/Q2RJ4K66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50370258 (TROLEANDOMYCIN | Triacetyloleandomycin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | DrugBank Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human Cytochrome P450 3A4 using BFC [7-benzyloxy-4-trifluoromethylcoumarin] after 30 minutes | J Med Chem 46: 3778-81 (2003) Article DOI: 10.1021/jm034111v BindingDB Entry DOI: 10.7270/Q2RJ4K66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50131898 ((E)-N-[1-((S)-4-Fluoro-3-morpholin-4-yl-phenyl)-et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human Cytochrome P450 3A4 with BFC [7-benzyloxy-4-trifluoromethylcoumarin] after 15 minutes | J Med Chem 46: 3778-81 (2003) Article DOI: 10.1021/jm034111v BindingDB Entry DOI: 10.7270/Q2RJ4K66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50130610 ((E)-N-[(S)-1-(3-Morpholin-4-yl-phenyl)-ethyl]-3-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human Cytochrome P450 3A4 with BFC [7-benzyloxy-4-trifluoromethylcoumarin] after 45 minutes | J Med Chem 46: 3778-81 (2003) Article DOI: 10.1021/jm034111v BindingDB Entry DOI: 10.7270/Q2RJ4K66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50130610 ((E)-N-[(S)-1-(3-Morpholin-4-yl-phenyl)-ethyl]-3-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human Cytochrome P450 3A4 with BFC [7-benzyloxy-4-trifluoromethylcoumarin] | J Med Chem 46: 3778-81 (2003) Article DOI: 10.1021/jm034111v BindingDB Entry DOI: 10.7270/Q2RJ4K66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50130610 ((E)-N-[(S)-1-(3-Morpholin-4-yl-phenyl)-ethyl]-3-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human Cytochrome P450 3A4 using BFC [7-benzyloxy-4-trifluoromethylcoumarin] after 30 minutes | J Med Chem 46: 3778-81 (2003) Article DOI: 10.1021/jm034111v BindingDB Entry DOI: 10.7270/Q2RJ4K66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50370258 (TROLEANDOMYCIN | Triacetyloleandomycin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | DrugBank Article PubMed | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human Cytochrome P450 3A4 with BFC [7-benzyloxy-4-trifluoromethylcoumarin] after 15 minutes | J Med Chem 46: 3778-81 (2003) Article DOI: 10.1021/jm034111v BindingDB Entry DOI: 10.7270/Q2RJ4K66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50370258 (TROLEANDOMYCIN | Triacetyloleandomycin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | DrugBank Article PubMed | n/a | n/a | 6.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human Cytochrome P450 3A4 with BFC [7-benzyloxy-4-trifluoromethylcoumarin] after 5 minutes | J Med Chem 46: 3778-81 (2003) Article DOI: 10.1021/jm034111v BindingDB Entry DOI: 10.7270/Q2RJ4K66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50130610 ((E)-N-[(S)-1-(3-Morpholin-4-yl-phenyl)-ethyl]-3-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human Cytochrome P450 3A4 with BFC [7-benzyloxy-4-trifluoromethylcoumarin] after 15 minutes | J Med Chem 46: 3778-81 (2003) Article DOI: 10.1021/jm034111v BindingDB Entry DOI: 10.7270/Q2RJ4K66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50130610 ((E)-N-[(S)-1-(3-Morpholin-4-yl-phenyl)-ethyl]-3-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human Cytochrome P450 3A4 with Benzoylresorufin | J Med Chem 46: 3778-81 (2003) Article DOI: 10.1021/jm034111v BindingDB Entry DOI: 10.7270/Q2RJ4K66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50130610 ((E)-N-[(S)-1-(3-Morpholin-4-yl-phenyl)-ethyl]-3-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human Cytochrome P450 1A2 | J Med Chem 46: 3778-81 (2003) Article DOI: 10.1021/jm034111v BindingDB Entry DOI: 10.7270/Q2RJ4K66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50130610 ((E)-N-[(S)-1-(3-Morpholin-4-yl-phenyl)-ethyl]-3-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human Cytochrome P450 3A4 with BFC [7-benzyloxy-4-trifluoromethylcoumarin] after 5 minutes | J Med Chem 46: 3778-81 (2003) Article DOI: 10.1021/jm034111v BindingDB Entry DOI: 10.7270/Q2RJ4K66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50130610 ((E)-N-[(S)-1-(3-Morpholin-4-yl-phenyl)-ethyl]-3-ph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human Cytochrome P450 2D6 | J Med Chem 46: 3778-81 (2003) Article DOI: 10.1021/jm034111v BindingDB Entry DOI: 10.7270/Q2RJ4K66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Homo sapiens (Human)) | BDBM50130610 ((E)-N-[(S)-1-(3-Morpholin-4-yl-phenyl)-ethyl]-3-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Effective concentration against Potassium voltage gated channel KQT-like subfamily, member 2 expressed in HEK 293 cells | J Med Chem 46: 3778-81 (2003) Article DOI: 10.1021/jm034111v BindingDB Entry DOI: 10.7270/Q2RJ4K66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Homo sapiens (Human)) | BDBM50131898 ((E)-N-[1-((S)-4-Fluoro-3-morpholin-4-yl-phenyl)-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Effective concentration against Potassium voltage gated channel KQT-like subfamily, member 2 expressed in HEK 293 cells | J Med Chem 46: 3778-81 (2003) Article DOI: 10.1021/jm034111v BindingDB Entry DOI: 10.7270/Q2RJ4K66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Homo sapiens (Human)) | BDBM50131898 ((E)-N-[1-((S)-4-Fluoro-3-morpholin-4-yl-phenyl)-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Effective concentration against Potassium voltage gated channel, KQT-like subfamily, member 2 expressed in SH-SY5Y human neuroblastoma cells | J Med Chem 46: 3778-81 (2003) Article DOI: 10.1021/jm034111v BindingDB Entry DOI: 10.7270/Q2RJ4K66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Homo sapiens (Human)) | BDBM50130610 ((E)-N-[(S)-1-(3-Morpholin-4-yl-phenyl)-ethyl]-3-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 690 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Effective concentration against Potassium voltage gated channel, KQT-like subfamily, member 2 expressed in SH-SY5Y human neuroblastoma cells | J Med Chem 46: 3778-81 (2003) Article DOI: 10.1021/jm034111v BindingDB Entry DOI: 10.7270/Q2RJ4K66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||