| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 3A4 |
|---|
| Ligand | BDBM50131898 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_51926 (CHEMBL663574) |
|---|
| IC50 | 21000±n/a nM |
|---|
| Citation |  Wu, YJ; Davis, CD; Dworetzky, S; Fitzpatrick, WC; Harden, D; He, H; Knox, RJ; Newton, AE; Philip, T; Polson, C; Sivarao, DV; Sun, LQ; Tertyshnikova, S; Weaver, D; Yeola, S; Zoeckler, M; Sinz, MW Fluorine substitution can block CYP3A4 metabolism-dependent inhibition: identification of (S)-N-[1-(4-fluoro-3- morpholin-4-ylphenyl)ethyl]-3- (4-fluorophenyl)acrylamide as an orally bioavailable KCNQ2 opener devoid of CYP3A4 metabolism-dependent inhibition. J Med Chem46:3778-81 (2003) [PubMed] Article Wu, YJ; Davis, CD; Dworetzky, S; Fitzpatrick, WC; Harden, D; He, H; Knox, RJ; Newton, AE; Philip, T; Polson, C; Sivarao, DV; Sun, LQ; Tertyshnikova, S; Weaver, D; Yeola, S; Zoeckler, M; Sinz, MW Fluorine substitution can block CYP3A4 metabolism-dependent inhibition: identification of (S)-N-[1-(4-fluoro-3- morpholin-4-ylphenyl)ethyl]-3- (4-fluorophenyl)acrylamide as an orally bioavailable KCNQ2 opener devoid of CYP3A4 metabolism-dependent inhibition. J Med Chem46:3778-81 (2003) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 3A4 |
|---|
| Name: | Cytochrome P450 3A4 |
|---|
| Synonyms: | Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 57349.57 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 503 |
|---|
| Sequence: | MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMF
DMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISI
AEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYS
MDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICV
FPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSI
IFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVV
NETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFS
KKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLG
GLLQPEKPVVLKVESRDGTVSGA
|
|
|
|---|
| BDBM50131898 |
|---|
| n/a |
|---|
| Name | BDBM50131898 |
|---|
| Synonyms: | (E)-N-[1-((S)-4-Fluoro-3-morpholin-4-yl-phenyl)-ethyl]-3-(4-fluoro-phenyl)-acrylamide | (S,E)-N-(1-(4-fluoro-3-morpholinophenyl)ethyl)-3-(4-fluorophenyl)acrylamide | CHEMBL100379 | N-[1-(4-Fluoro-3-morpholin-4-yl-phenyl)-ethyl]-3-(4-fluoro-phenyl)-acrylamide |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C21H22F2N2O2 |
|---|
| Mol. Mass. | 372.4084 |
|---|
| SMILES | C[C@H](NC(=O)\C=C\c1ccc(F)cc1)c1ccc(F)c(c1)N1CCOCC1 |
|---|
| Structure | 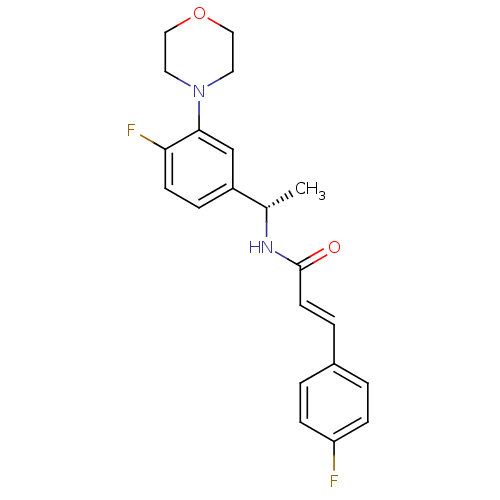
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Wu, YJ; Davis, CD; Dworetzky, S; Fitzpatrick, WC; Harden, D; He, H; Knox, RJ; Newton, AE; Philip, T; Polson, C; Sivarao, DV; Sun, LQ; Tertyshnikova, S; Weaver, D; Yeola, S; Zoeckler, M; Sinz, MW Fluorine substitution can block CYP3A4 metabolism-dependent inhibition: identification of (S)-N-[1-(4-fluoro-3- morpholin-4-ylphenyl)ethyl]-3- (4-fluorophenyl)acrylamide as an orally bioavailable KCNQ2 opener devoid of CYP3A4 metabolism-dependent inhibition. J Med Chem46:3778-81 (2003) [PubMed] Article
Wu, YJ; Davis, CD; Dworetzky, S; Fitzpatrick, WC; Harden, D; He, H; Knox, RJ; Newton, AE; Philip, T; Polson, C; Sivarao, DV; Sun, LQ; Tertyshnikova, S; Weaver, D; Yeola, S; Zoeckler, M; Sinz, MW Fluorine substitution can block CYP3A4 metabolism-dependent inhibition: identification of (S)-N-[1-(4-fluoro-3- morpholin-4-ylphenyl)ethyl]-3- (4-fluorophenyl)acrylamide as an orally bioavailable KCNQ2 opener devoid of CYP3A4 metabolism-dependent inhibition. J Med Chem46:3778-81 (2003) [PubMed] Article