

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50130610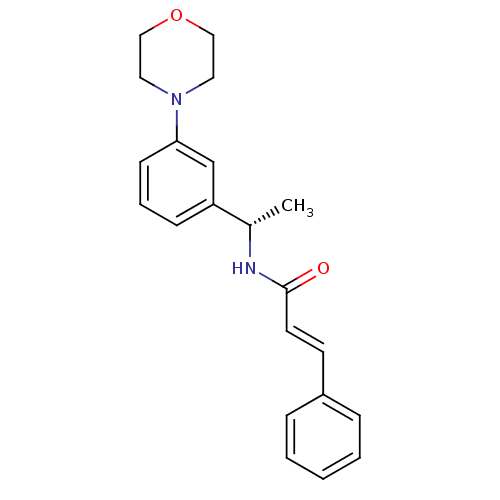 ((E)-N-[(S)-1-(3-Morpholin-4-yl-phenyl)-ethyl]-3-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human Cytochrome P450 2C9 | J Med Chem 46: 3778-81 (2003) Article DOI: 10.1021/jm034111v BindingDB Entry DOI: 10.7270/Q2RJ4K66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50130610 ((E)-N-[(S)-1-(3-Morpholin-4-yl-phenyl)-ethyl]-3-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human Cytochrome P450 19A1 | J Med Chem 46: 3778-81 (2003) Article DOI: 10.1021/jm034111v BindingDB Entry DOI: 10.7270/Q2RJ4K66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50370258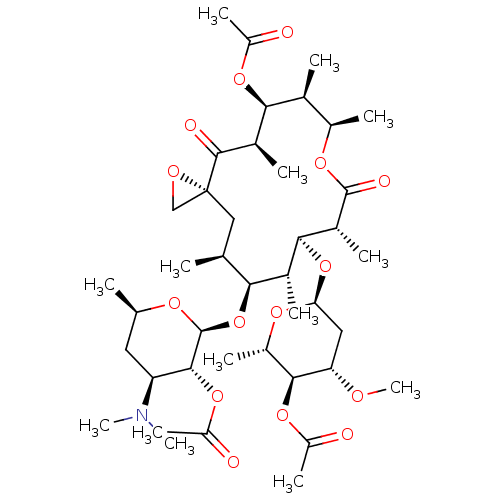 (TROLEANDOMYCIN | Triacetyloleandomycin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | DrugBank Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human Cytochrome P450 3A4 with BFC [7-benzyloxy-4-trifluoromethylcoumarin] after 45 minutes | J Med Chem 46: 3778-81 (2003) Article DOI: 10.1021/jm034111v BindingDB Entry DOI: 10.7270/Q2RJ4K66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50131898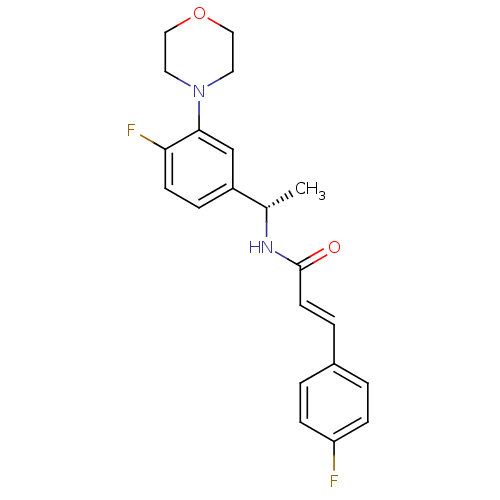 ((E)-N-[1-((S)-4-Fluoro-3-morpholin-4-yl-phenyl)-et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human Cytochrome P450 3A4 with BFC [7-benzyloxy-4-trifluoromethylcoumarin] after 5 minutes | J Med Chem 46: 3778-81 (2003) Article DOI: 10.1021/jm034111v BindingDB Entry DOI: 10.7270/Q2RJ4K66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50131898 ((E)-N-[1-((S)-4-Fluoro-3-morpholin-4-yl-phenyl)-et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human Cytochrome P450 3A4 using BFC [7-benzyloxy-4-trifluoromethylcoumarin] after 30 minutes | J Med Chem 46: 3778-81 (2003) Article DOI: 10.1021/jm034111v BindingDB Entry DOI: 10.7270/Q2RJ4K66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50131898 ((E)-N-[1-((S)-4-Fluoro-3-morpholin-4-yl-phenyl)-et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human Cytochrome P450 3A4 with BFC [7-benzyloxy-4-trifluoromethylcoumarin] after 45 minutes | J Med Chem 46: 3778-81 (2003) Article DOI: 10.1021/jm034111v BindingDB Entry DOI: 10.7270/Q2RJ4K66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50430241 (CHEMBL2332488) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of 5HT3 receptor (unknown origin) | Bioorg Med Chem Lett 23: 1684-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.070 BindingDB Entry DOI: 10.7270/Q2M046SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50370258 (TROLEANDOMYCIN | Triacetyloleandomycin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | DrugBank Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human Cytochrome P450 3A4 using BFC [7-benzyloxy-4-trifluoromethylcoumarin] after 30 minutes | J Med Chem 46: 3778-81 (2003) Article DOI: 10.1021/jm034111v BindingDB Entry DOI: 10.7270/Q2RJ4K66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50131898 ((E)-N-[1-((S)-4-Fluoro-3-morpholin-4-yl-phenyl)-et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human Cytochrome P450 3A4 with BFC [7-benzyloxy-4-trifluoromethylcoumarin] after 15 minutes | J Med Chem 46: 3778-81 (2003) Article DOI: 10.1021/jm034111v BindingDB Entry DOI: 10.7270/Q2RJ4K66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50130610 ((E)-N-[(S)-1-(3-Morpholin-4-yl-phenyl)-ethyl]-3-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human Cytochrome P450 3A4 with BFC [7-benzyloxy-4-trifluoromethylcoumarin] after 45 minutes | J Med Chem 46: 3778-81 (2003) Article DOI: 10.1021/jm034111v BindingDB Entry DOI: 10.7270/Q2RJ4K66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50130610 ((E)-N-[(S)-1-(3-Morpholin-4-yl-phenyl)-ethyl]-3-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human Cytochrome P450 3A4 with BFC [7-benzyloxy-4-trifluoromethylcoumarin] | J Med Chem 46: 3778-81 (2003) Article DOI: 10.1021/jm034111v BindingDB Entry DOI: 10.7270/Q2RJ4K66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50130610 ((E)-N-[(S)-1-(3-Morpholin-4-yl-phenyl)-ethyl]-3-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human Cytochrome P450 3A4 using BFC [7-benzyloxy-4-trifluoromethylcoumarin] after 30 minutes | J Med Chem 46: 3778-81 (2003) Article DOI: 10.1021/jm034111v BindingDB Entry DOI: 10.7270/Q2RJ4K66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50370258 (TROLEANDOMYCIN | Triacetyloleandomycin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | DrugBank Article PubMed | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human Cytochrome P450 3A4 with BFC [7-benzyloxy-4-trifluoromethylcoumarin] after 15 minutes | J Med Chem 46: 3778-81 (2003) Article DOI: 10.1021/jm034111v BindingDB Entry DOI: 10.7270/Q2RJ4K66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50370258 (TROLEANDOMYCIN | Triacetyloleandomycin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | DrugBank Article PubMed | n/a | n/a | 6.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human Cytochrome P450 3A4 with BFC [7-benzyloxy-4-trifluoromethylcoumarin] after 5 minutes | J Med Chem 46: 3778-81 (2003) Article DOI: 10.1021/jm034111v BindingDB Entry DOI: 10.7270/Q2RJ4K66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50130610 ((E)-N-[(S)-1-(3-Morpholin-4-yl-phenyl)-ethyl]-3-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human Cytochrome P450 3A4 with BFC [7-benzyloxy-4-trifluoromethylcoumarin] after 15 minutes | J Med Chem 46: 3778-81 (2003) Article DOI: 10.1021/jm034111v BindingDB Entry DOI: 10.7270/Q2RJ4K66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50130610 ((E)-N-[(S)-1-(3-Morpholin-4-yl-phenyl)-ethyl]-3-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human Cytochrome P450 3A4 with Benzoylresorufin | J Med Chem 46: 3778-81 (2003) Article DOI: 10.1021/jm034111v BindingDB Entry DOI: 10.7270/Q2RJ4K66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50130610 ((E)-N-[(S)-1-(3-Morpholin-4-yl-phenyl)-ethyl]-3-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human Cytochrome P450 1A2 | J Med Chem 46: 3778-81 (2003) Article DOI: 10.1021/jm034111v BindingDB Entry DOI: 10.7270/Q2RJ4K66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50130610 ((E)-N-[(S)-1-(3-Morpholin-4-yl-phenyl)-ethyl]-3-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human Cytochrome P450 3A4 with BFC [7-benzyloxy-4-trifluoromethylcoumarin] after 5 minutes | J Med Chem 46: 3778-81 (2003) Article DOI: 10.1021/jm034111v BindingDB Entry DOI: 10.7270/Q2RJ4K66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50130610 ((E)-N-[(S)-1-(3-Morpholin-4-yl-phenyl)-ethyl]-3-ph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human Cytochrome P450 2D6 | J Med Chem 46: 3778-81 (2003) Article DOI: 10.1021/jm034111v BindingDB Entry DOI: 10.7270/Q2RJ4K66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2/3 (Homo sapiens (Human)) | BDBM50130610 ((E)-N-[(S)-1-(3-Morpholin-4-yl-phenyl)-ethyl]-3-ph...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 690 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Effect on resting membrane potential in SH-SY5Y human neuroblastoma cells expressing native KCNQ channels | J Med Chem 46: 3197-200 (2003) Article DOI: 10.1021/jm034073f BindingDB Entry DOI: 10.7270/Q2D21X11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Mus musculus) | BDBM50130610 ((E)-N-[(S)-1-(3-Morpholin-4-yl-phenyl)-ethyl]-3-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Induced current in Xenopus laevis oocytes expressing cloned mKCNQ2 channels | J Med Chem 46: 3197-200 (2003) Article DOI: 10.1021/jm034073f BindingDB Entry DOI: 10.7270/Q2D21X11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Mus musculus) | BDBM50143557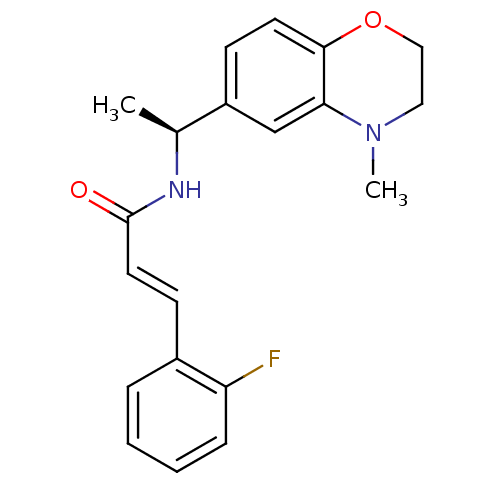 ((E)-3-(2-Fluoro-phenyl)-N-[1-((S)-4-methyl-3,4-dih...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 940 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Whole-cell patch-clamp on recombinant mouse KCNQ2 channels expressed in HEK 293 cells at -40 mV | Bioorg Med Chem Lett 14: 1991-5 (2004) Article DOI: 10.1016/j.bmcl.2004.01.069 BindingDB Entry DOI: 10.7270/Q2MG7NZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Mus musculus) | BDBM50130610 ((E)-N-[(S)-1-(3-Morpholin-4-yl-phenyl)-ethyl]-3-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.28E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Whole-cell patch-clamp on recombinant mouse KCNQ2 channels expressed in HEK 293 cells at -40 mV | Bioorg Med Chem Lett 14: 1991-5 (2004) Article DOI: 10.1016/j.bmcl.2004.01.069 BindingDB Entry DOI: 10.7270/Q2MG7NZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Mus musculus) | BDBM50143558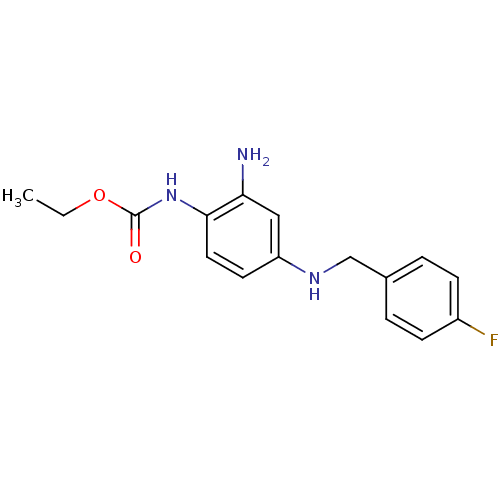 (CHEMBL41355 | EZOGABINE | N-(2-amino-4-(4-fluorobe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Whole-cell patch-clamp on recombinant mouse KCNQ2 channels expressed in HEK 293 cells at -40 mV | Bioorg Med Chem Lett 14: 1991-5 (2004) Article DOI: 10.1016/j.bmcl.2004.01.069 BindingDB Entry DOI: 10.7270/Q2MG7NZ3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Mus musculus) | BDBM50143560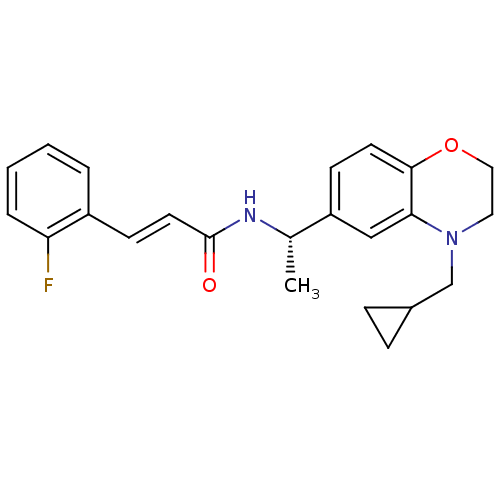 ((E)-N-[(S)-1-(4-Cyclopropylmethyl-3,4-dihydro-2H-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Whole-cell patch-clamp on recombinant mouse KCNQ2 channels expressed in HEK 293 cells at -40 mV | Bioorg Med Chem Lett 14: 1991-5 (2004) Article DOI: 10.1016/j.bmcl.2004.01.069 BindingDB Entry DOI: 10.7270/Q2MG7NZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Mus musculus) | BDBM50143559 ((E)-N-[1-((S)-4-Ethyl-3,4-dihydro-2H-benzo[1,4]oxa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Whole-cell patch-clamp on recombinant mouse KCNQ2 channels expressed in HEK 293 cells at -40 mV | Bioorg Med Chem Lett 14: 1991-5 (2004) Article DOI: 10.1016/j.bmcl.2004.01.069 BindingDB Entry DOI: 10.7270/Q2MG7NZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Mus musculus) | BDBM50143561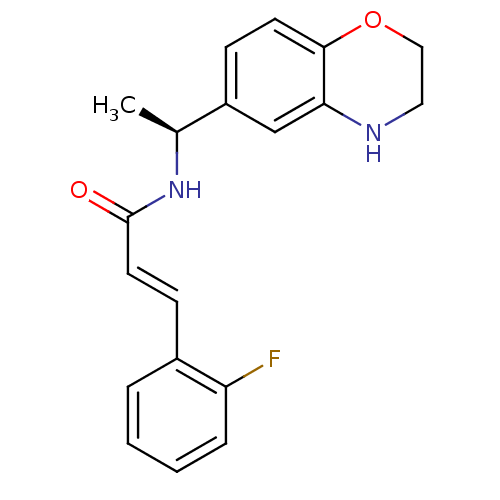 ((E)-N-[(S)-1-(3,4-Dihydro-2H-benzo[1,4]oxazin-6-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Whole-cell patch-clamp on recombinant mouse KCNQ2 channels expressed in HEK 293 cells at -40 mV | Bioorg Med Chem Lett 14: 1991-5 (2004) Article DOI: 10.1016/j.bmcl.2004.01.069 BindingDB Entry DOI: 10.7270/Q2MG7NZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Mus musculus) | BDBM50142346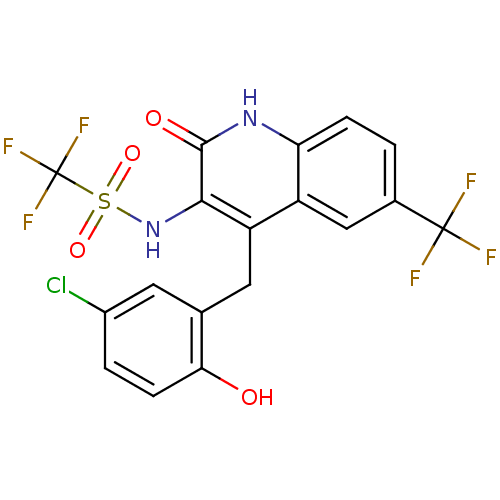 (CHEMBL41078 | N-[4-(5-Chloro-2-hydroxy-benzyl)-2-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 663 | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Increased outward current at -40 mV mediated by mouse KCNQ2 channel expressed in Xenopus laevis oocytes | Bioorg Med Chem Lett 14: 1615-8 (2004) Article DOI: 10.1016/j.bmcl.2004.01.073 BindingDB Entry DOI: 10.7270/Q20V8C7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Homo sapiens (Human)) | BDBM50130610 ((E)-N-[(S)-1-(3-Morpholin-4-yl-phenyl)-ethyl]-3-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Effective concentration against Potassium voltage gated channel KQT-like subfamily, member 2 expressed in HEK 293 cells | J Med Chem 46: 3778-81 (2003) Article DOI: 10.1021/jm034111v BindingDB Entry DOI: 10.7270/Q2RJ4K66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Homo sapiens (Human)) | BDBM50131898 ((E)-N-[1-((S)-4-Fluoro-3-morpholin-4-yl-phenyl)-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Effective concentration against Potassium voltage gated channel KQT-like subfamily, member 2 expressed in HEK 293 cells | J Med Chem 46: 3778-81 (2003) Article DOI: 10.1021/jm034111v BindingDB Entry DOI: 10.7270/Q2RJ4K66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Homo sapiens (Human)) | BDBM50131898 ((E)-N-[1-((S)-4-Fluoro-3-morpholin-4-yl-phenyl)-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Effective concentration against Potassium voltage gated channel, KQT-like subfamily, member 2 expressed in SH-SY5Y human neuroblastoma cells | J Med Chem 46: 3778-81 (2003) Article DOI: 10.1021/jm034111v BindingDB Entry DOI: 10.7270/Q2RJ4K66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Homo sapiens (Human)) | BDBM50130610 ((E)-N-[(S)-1-(3-Morpholin-4-yl-phenyl)-ethyl]-3-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 690 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Effective concentration against Potassium voltage gated channel, KQT-like subfamily, member 2 expressed in SH-SY5Y human neuroblastoma cells | J Med Chem 46: 3778-81 (2003) Article DOI: 10.1021/jm034111v BindingDB Entry DOI: 10.7270/Q2RJ4K66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Mus musculus) | BDBM50442468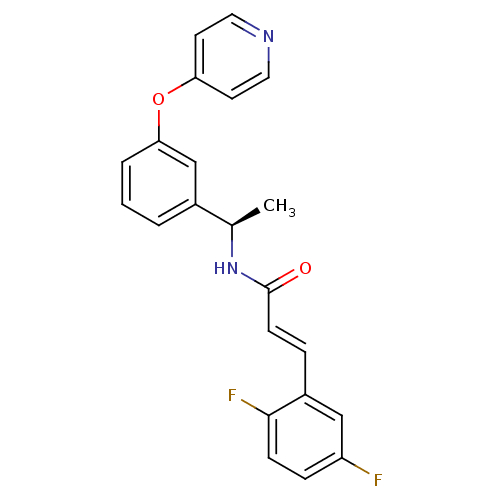 (CHEMBL2440364) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Induction of mouse KCNQ2 expressed in HEK293 cells at -40 mV holding potential by whole-cell patch clamp assay | Bioorg Med Chem Lett 23: 6188-91 (2013) Article DOI: 10.1016/j.bmcl.2013.08.092 BindingDB Entry DOI: 10.7270/Q2F76F0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Mus musculus) | BDBM50442469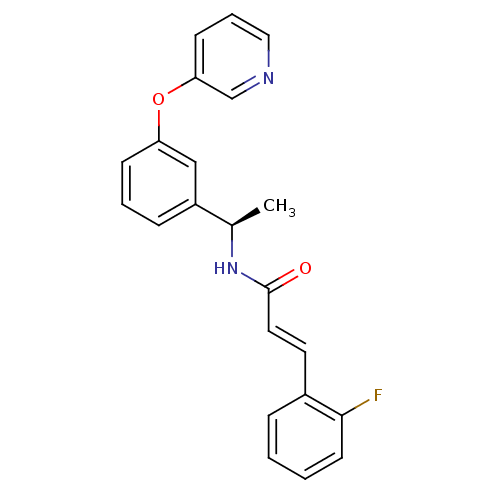 (CHEMBL2440362) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Induction of mouse KCNQ2 expressed in HEK293 cells at -40 mV holding potential by whole-cell patch clamp assay | Bioorg Med Chem Lett 23: 6188-91 (2013) Article DOI: 10.1016/j.bmcl.2013.08.092 BindingDB Entry DOI: 10.7270/Q2F76F0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Mus musculus) | BDBM50130610 ((E)-N-[(S)-1-(3-Morpholin-4-yl-phenyl)-ethyl]-3-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Induction of mouse KCNQ2 expressed in HEK293 cells at -40 mV holding potential by whole-cell patch clamp assay | Bioorg Med Chem Lett 23: 6188-91 (2013) Article DOI: 10.1016/j.bmcl.2013.08.092 BindingDB Entry DOI: 10.7270/Q2F76F0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Mus musculus) | BDBM50143558 (CHEMBL41355 | EZOGABINE | N-(2-amino-4-(4-fluorobe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Induction of mouse KCNQ2 expressed in HEK293 cells at -40 mV holding potential by whole-cell patch clamp assay | Bioorg Med Chem Lett 23: 6188-91 (2013) Article DOI: 10.1016/j.bmcl.2013.08.092 BindingDB Entry DOI: 10.7270/Q2F76F0Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Mus musculus) | BDBM50143560 ((E)-N-[(S)-1-(4-Cyclopropylmethyl-3,4-dihydro-2H-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Induction of mouse KCNQ2 expressed in HEK293 cells at -40 mV holding potential by whole-cell patch clamp assay | Bioorg Med Chem Lett 23: 6188-91 (2013) Article DOI: 10.1016/j.bmcl.2013.08.092 BindingDB Entry DOI: 10.7270/Q2F76F0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Mus musculus) | BDBM50410077 (CHEMBL180886) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Induction of mouse KCNQ2 expressed in HEK293 cells at -40 mV holding potential by whole-cell patch clamp assay | Bioorg Med Chem Lett 23: 6188-91 (2013) Article DOI: 10.1016/j.bmcl.2013.08.092 BindingDB Entry DOI: 10.7270/Q2F76F0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Mus musculus) | BDBM50410081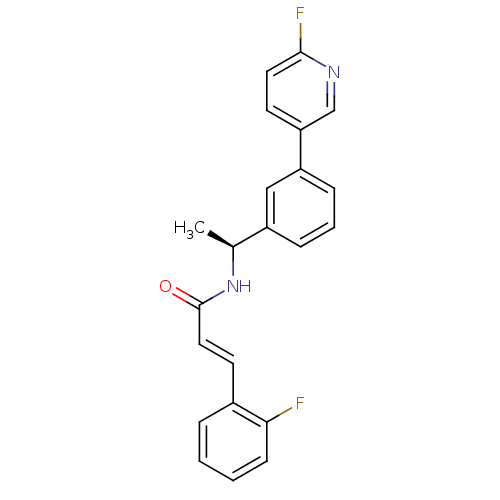 (CHEMBL361184) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Induction of mouse KCNQ2 expressed in HEK293 cells at -40 mV holding potential by whole-cell patch clamp assay | Bioorg Med Chem Lett 23: 6188-91 (2013) Article DOI: 10.1016/j.bmcl.2013.08.092 BindingDB Entry DOI: 10.7270/Q2F76F0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Mus musculus) | BDBM50442470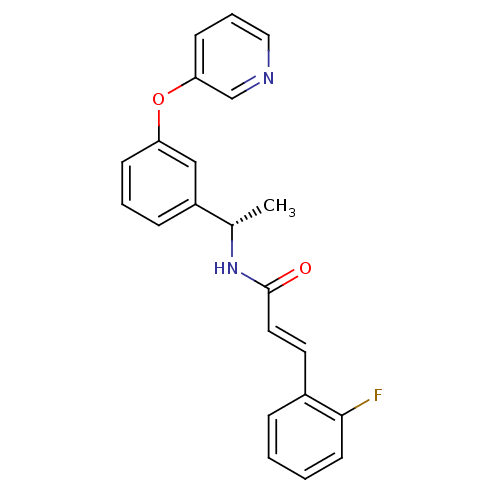 (CHEMBL401942) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Induction of mouse KCNQ2 expressed in HEK293 cells at -40 mV holding potential by whole-cell patch clamp assay | Bioorg Med Chem Lett 23: 6188-91 (2013) Article DOI: 10.1016/j.bmcl.2013.08.092 BindingDB Entry DOI: 10.7270/Q2F76F0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Mus musculus) | BDBM50442471 (CHEMBL2440363) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Induction of mouse KCNQ2 expressed in HEK293 cells at -40 mV holding potential by whole-cell patch clamp assay | Bioorg Med Chem Lett 23: 6188-91 (2013) Article DOI: 10.1016/j.bmcl.2013.08.092 BindingDB Entry DOI: 10.7270/Q2F76F0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50430230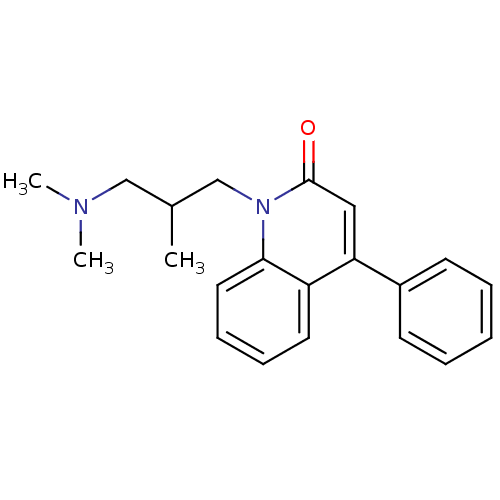 (CHEMBL2332484) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Agonist activity at rat alpha7 nAChR expressed in HEK293 cells assessed as calcium ion influx by FRET assay | Bioorg Med Chem Lett 23: 1684-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.070 BindingDB Entry DOI: 10.7270/Q2M046SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50430231 (CHEMBL2332486) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Agonist activity at rat alpha7 nAChR expressed in HEK293 cells assessed as calcium ion influx by FRET assay | Bioorg Med Chem Lett 23: 1684-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.070 BindingDB Entry DOI: 10.7270/Q2M046SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50430232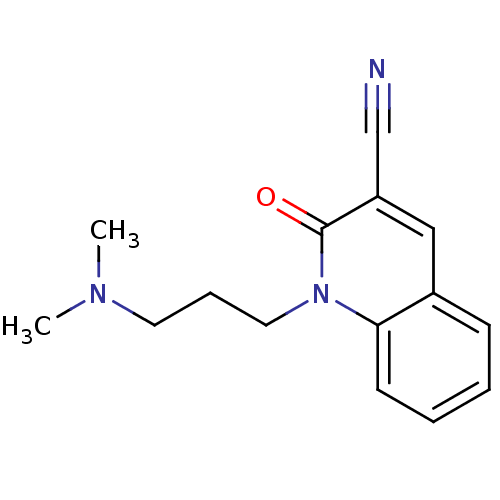 (CHEMBL2332485) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Agonist activity at rat alpha7 nAChR expressed in HEK293 cells assessed as calcium ion influx by FRET assay | Bioorg Med Chem Lett 23: 1684-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.070 BindingDB Entry DOI: 10.7270/Q2M046SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50430233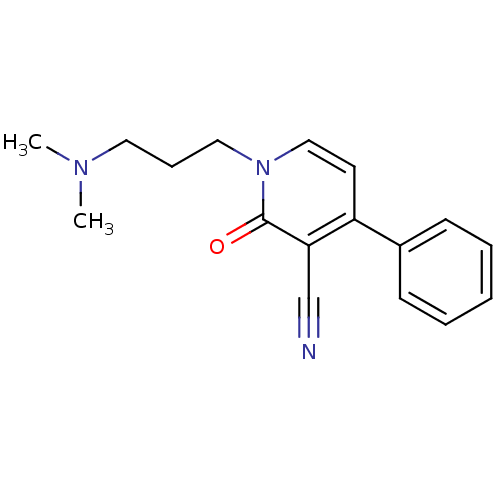 (CHEMBL2332487) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Agonist activity at rat alpha7 nAChR expressed in HEK293 cells assessed as calcium ion influx by FRET assay | Bioorg Med Chem Lett 23: 1684-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.070 BindingDB Entry DOI: 10.7270/Q2M046SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50430234 (CHEMBL2332491) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Agonist activity at rat alpha7 nAChR expressed in HEK293 cells assessed as calcium ion influx by FRET assay | Bioorg Med Chem Lett 23: 1684-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.070 BindingDB Entry DOI: 10.7270/Q2M046SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50430235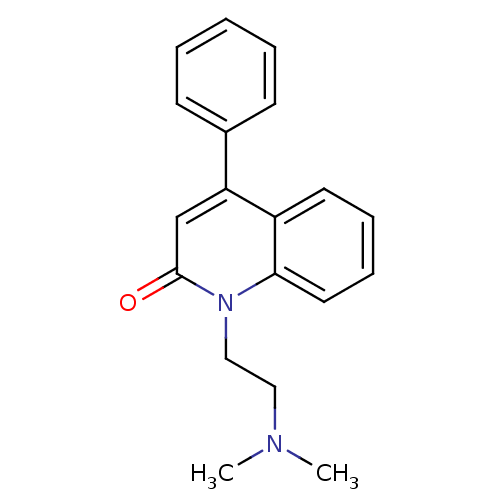 (CHEMBL2332490) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Agonist activity at rat alpha7 nAChR expressed in HEK293 cells assessed as calcium ion influx by FRET assay | Bioorg Med Chem Lett 23: 1684-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.070 BindingDB Entry DOI: 10.7270/Q2M046SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50430236 (CHEMBL2332482) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Agonist activity at rat alpha7 nAChR expressed in HEK293 cells assessed as calcium ion influx by FRET assay | Bioorg Med Chem Lett 23: 1684-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.070 BindingDB Entry DOI: 10.7270/Q2M046SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50430237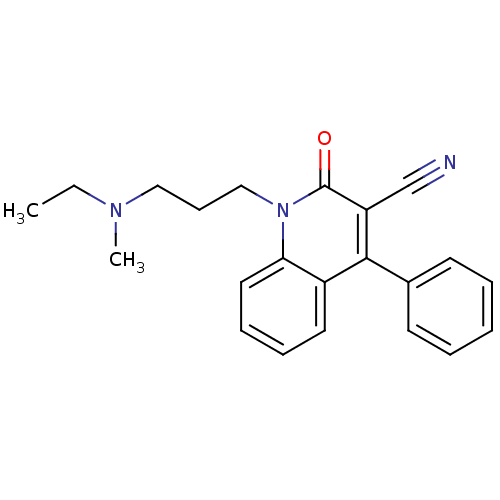 (CHEMBL2332483) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Agonist activity at rat alpha7 nAChR expressed in HEK293 cells assessed as calcium ion influx by FRET assay | Bioorg Med Chem Lett 23: 1684-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.070 BindingDB Entry DOI: 10.7270/Q2M046SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50430238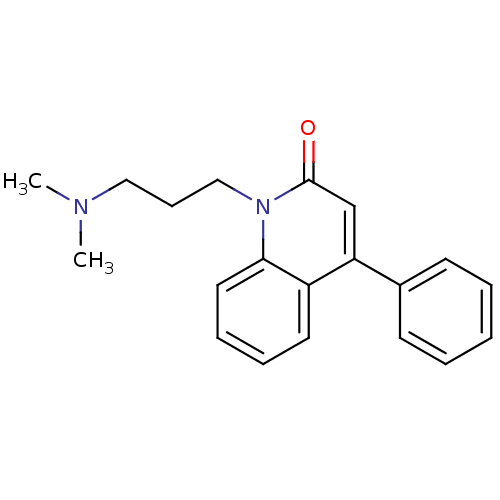 (CHEMBL2332489) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Agonist activity at rat alpha7 nAChR expressed in HEK293 cells assessed as calcium ion influx by FRET assay | Bioorg Med Chem Lett 23: 1684-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.070 BindingDB Entry DOI: 10.7270/Q2M046SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 83 total ) | Next | Last >> |