

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50150408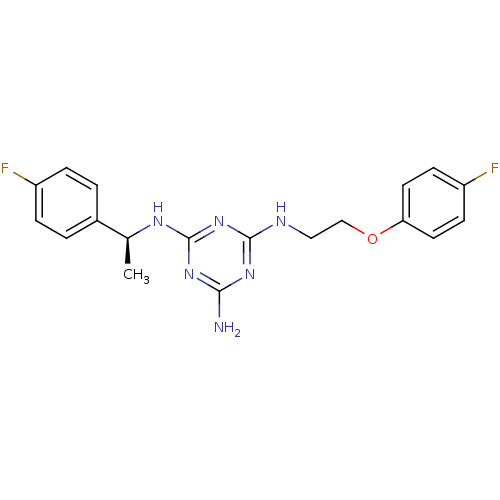 (CHEMBL182937 | N-[2-(4-Fluoro-phenoxy)-ethyl]-N''-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 7 receptor | Bioorg Med Chem Lett 14: 4245-8 (2004) Article DOI: 10.1016/j.bmcl.2004.06.008 BindingDB Entry DOI: 10.7270/Q2GM86RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50150413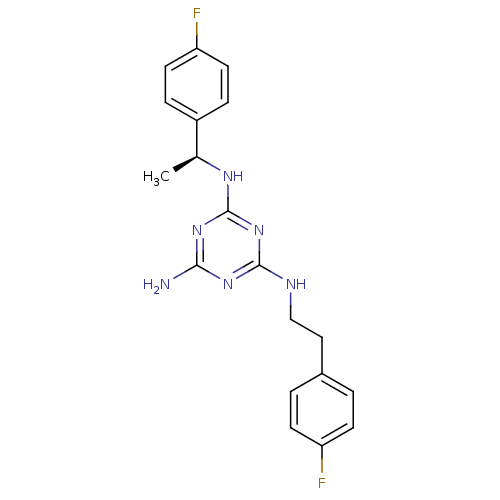 (CHEMBL413049 | N-[2-(4-Fluoro-phenyl)-ethyl]-N''-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 7 receptor | Bioorg Med Chem Lett 14: 4245-8 (2004) Article DOI: 10.1016/j.bmcl.2004.06.008 BindingDB Entry DOI: 10.7270/Q2GM86RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50150407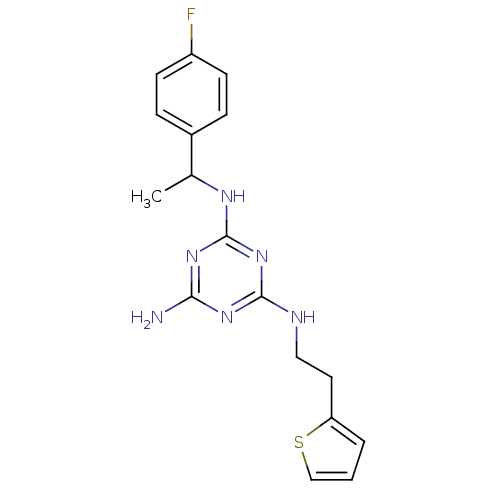 (CHEMBL180086 | N-[1-(4-Fluoro-phenyl)-ethyl]-N''-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 7 receptor | Bioorg Med Chem Lett 14: 4245-8 (2004) Article DOI: 10.1016/j.bmcl.2004.06.008 BindingDB Entry DOI: 10.7270/Q2GM86RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50150412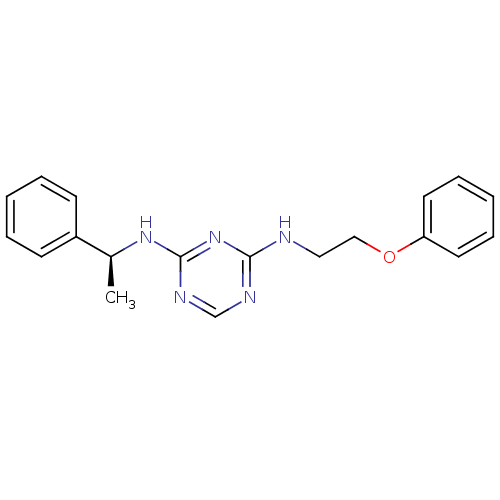 (CHEMBL183862 | N-(2-Phenoxy-ethyl)-N''-((S)-1-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 7 receptor | Bioorg Med Chem Lett 14: 4245-8 (2004) Article DOI: 10.1016/j.bmcl.2004.06.008 BindingDB Entry DOI: 10.7270/Q2GM86RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50150410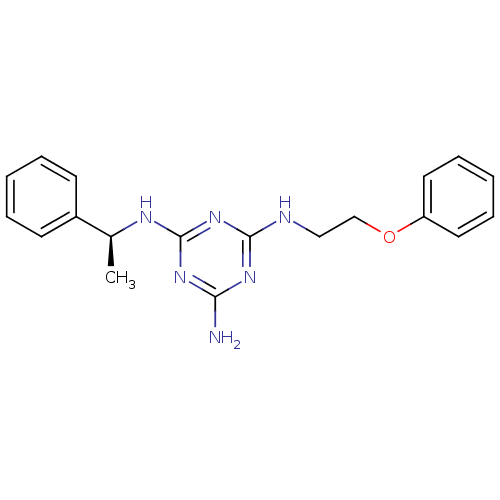 (CHEMBL182174 | N-(2-Phenoxy-ethyl)-N''-((S)-1-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 7 receptor | Bioorg Med Chem Lett 14: 4245-8 (2004) Article DOI: 10.1016/j.bmcl.2004.06.008 BindingDB Entry DOI: 10.7270/Q2GM86RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50150417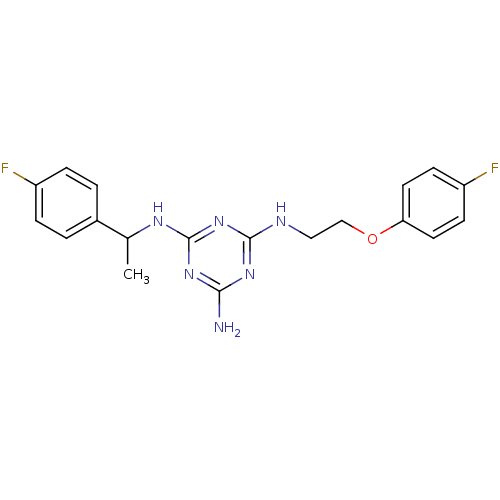 (CHEMBL182322 | N-[2-(4-Fluoro-phenoxy)-ethyl]-N''-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 7 receptor | Bioorg Med Chem Lett 14: 4245-8 (2004) Article DOI: 10.1016/j.bmcl.2004.06.008 BindingDB Entry DOI: 10.7270/Q2GM86RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50150405 (CHEMBL182797 | N-(2-Phenoxy-ethyl)-N''-(1-phenyl-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 7 receptor | Bioorg Med Chem Lett 14: 4245-8 (2004) Article DOI: 10.1016/j.bmcl.2004.06.008 BindingDB Entry DOI: 10.7270/Q2GM86RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50150421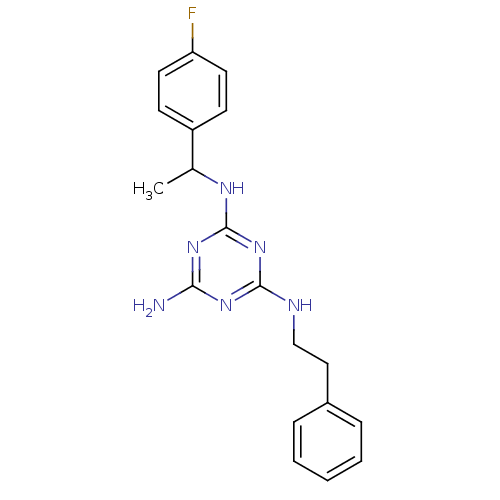 (CHEMBL183462 | N-[1-(4-Fluoro-phenyl)-ethyl]-N''-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 7 receptor | Bioorg Med Chem Lett 14: 4245-8 (2004) Article DOI: 10.1016/j.bmcl.2004.06.008 BindingDB Entry DOI: 10.7270/Q2GM86RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50150406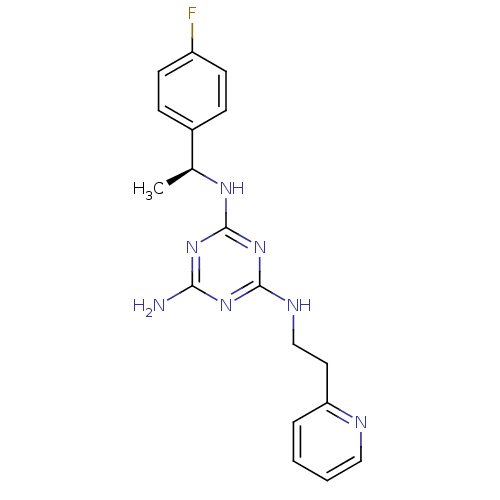 (CHEMBL182418 | N-[(S)-1-(4-Fluoro-phenyl)-ethyl]-N...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 7 receptor | Bioorg Med Chem Lett 14: 4245-8 (2004) Article DOI: 10.1016/j.bmcl.2004.06.008 BindingDB Entry DOI: 10.7270/Q2GM86RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50150420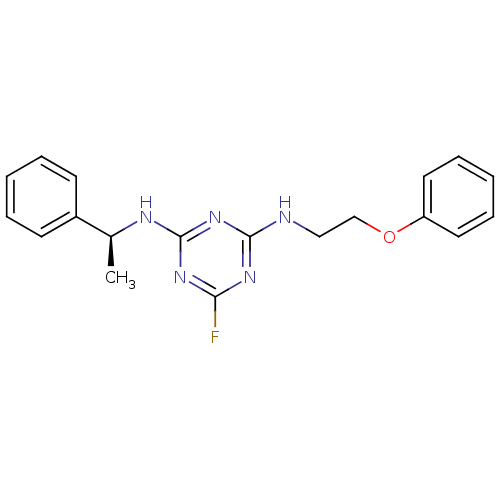 (6-Fluoro-N-(2-phenoxy-ethyl)-N''-((S)-1-phenyl-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 7 receptor | Bioorg Med Chem Lett 14: 4245-8 (2004) Article DOI: 10.1016/j.bmcl.2004.06.008 BindingDB Entry DOI: 10.7270/Q2GM86RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50150407 (CHEMBL180086 | N-[1-(4-Fluoro-phenyl)-ethyl]-N''-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 2C receptor | Bioorg Med Chem Lett 14: 4245-8 (2004) Article DOI: 10.1016/j.bmcl.2004.06.008 BindingDB Entry DOI: 10.7270/Q2GM86RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50150413 (CHEMBL413049 | N-[2-(4-Fluoro-phenyl)-ethyl]-N''-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 2C receptor | Bioorg Med Chem Lett 14: 4245-8 (2004) Article DOI: 10.1016/j.bmcl.2004.06.008 BindingDB Entry DOI: 10.7270/Q2GM86RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50150414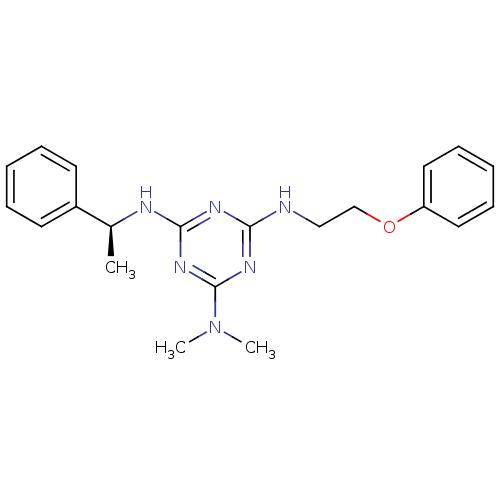 (CHEMBL182840 | N,N-Dimethyl-N''-(2-phenoxy-ethyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 7 receptor | Bioorg Med Chem Lett 14: 4245-8 (2004) Article DOI: 10.1016/j.bmcl.2004.06.008 BindingDB Entry DOI: 10.7270/Q2GM86RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50150411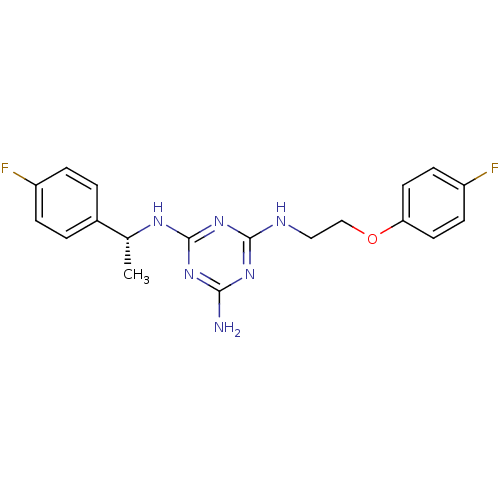 (CHEMBL180946 | N-[2-(4-Fluoro-phenoxy)-ethyl]-N''-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 7 receptor | Bioorg Med Chem Lett 14: 4245-8 (2004) Article DOI: 10.1016/j.bmcl.2004.06.008 BindingDB Entry DOI: 10.7270/Q2GM86RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50150409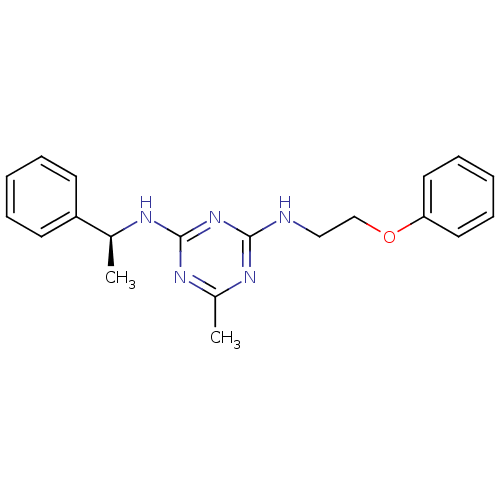 (6-Methyl-N-(2-phenoxy-ethyl)-N''-((S)-1-phenyl-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 7 receptor | Bioorg Med Chem Lett 14: 4245-8 (2004) Article DOI: 10.1016/j.bmcl.2004.06.008 BindingDB Entry DOI: 10.7270/Q2GM86RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50150416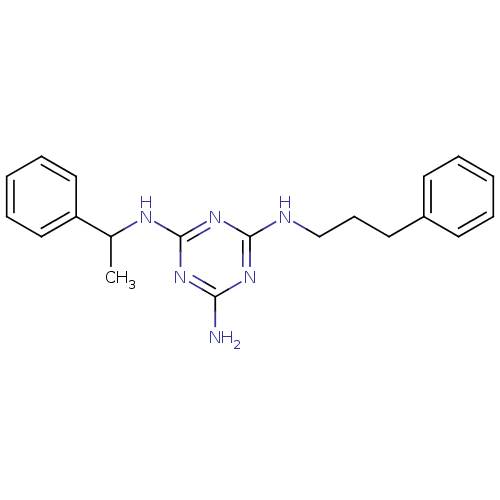 (CHEMBL182121 | N-(1-Phenyl-ethyl)-N''-(3-phenyl-pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 7 receptor | Bioorg Med Chem Lett 14: 4245-8 (2004) Article DOI: 10.1016/j.bmcl.2004.06.008 BindingDB Entry DOI: 10.7270/Q2GM86RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50150406 (CHEMBL182418 | N-[(S)-1-(4-Fluoro-phenyl)-ethyl]-N...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 2C receptor | Bioorg Med Chem Lett 14: 4245-8 (2004) Article DOI: 10.1016/j.bmcl.2004.06.008 BindingDB Entry DOI: 10.7270/Q2GM86RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50150413 (CHEMBL413049 | N-[2-(4-Fluoro-phenyl)-ethyl]-N''-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 172 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 6 receptor | Bioorg Med Chem Lett 14: 4245-8 (2004) Article DOI: 10.1016/j.bmcl.2004.06.008 BindingDB Entry DOI: 10.7270/Q2GM86RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50150407 (CHEMBL180086 | N-[1-(4-Fluoro-phenyl)-ethyl]-N''-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 6 receptor | Bioorg Med Chem Lett 14: 4245-8 (2004) Article DOI: 10.1016/j.bmcl.2004.06.008 BindingDB Entry DOI: 10.7270/Q2GM86RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50150408 (CHEMBL182937 | N-[2-(4-Fluoro-phenoxy)-ethyl]-N''-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 2C receptor | Bioorg Med Chem Lett 14: 4245-8 (2004) Article DOI: 10.1016/j.bmcl.2004.06.008 BindingDB Entry DOI: 10.7270/Q2GM86RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50150410 (CHEMBL182174 | N-(2-Phenoxy-ethyl)-N''-((S)-1-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 2C receptor | Bioorg Med Chem Lett 14: 4245-8 (2004) Article DOI: 10.1016/j.bmcl.2004.06.008 BindingDB Entry DOI: 10.7270/Q2GM86RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50150415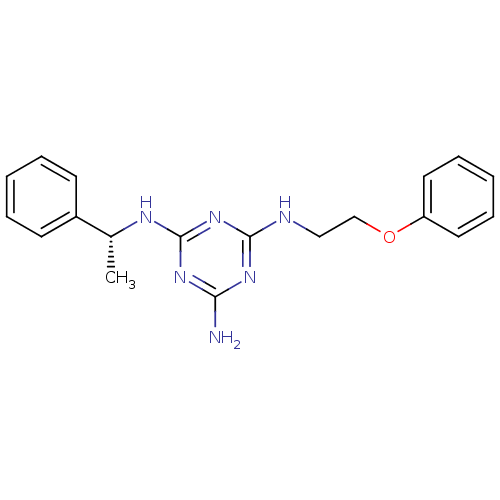 (CHEMBL360374 | N-(2-Phenoxy-ethyl)-N''-((R)-1-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 7 receptor | Bioorg Med Chem Lett 14: 4245-8 (2004) Article DOI: 10.1016/j.bmcl.2004.06.008 BindingDB Entry DOI: 10.7270/Q2GM86RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50150410 (CHEMBL182174 | N-(2-Phenoxy-ethyl)-N''-((S)-1-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 6 receptor | Bioorg Med Chem Lett 14: 4245-8 (2004) Article DOI: 10.1016/j.bmcl.2004.06.008 BindingDB Entry DOI: 10.7270/Q2GM86RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50150408 (CHEMBL182937 | N-[2-(4-Fluoro-phenoxy)-ethyl]-N''-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 6 receptor | Bioorg Med Chem Lett 14: 4245-8 (2004) Article DOI: 10.1016/j.bmcl.2004.06.008 BindingDB Entry DOI: 10.7270/Q2GM86RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50150406 (CHEMBL182418 | N-[(S)-1-(4-Fluoro-phenyl)-ethyl]-N...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 6 receptor | Bioorg Med Chem Lett 14: 4245-8 (2004) Article DOI: 10.1016/j.bmcl.2004.06.008 BindingDB Entry DOI: 10.7270/Q2GM86RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50150419 (CHEMBL185560 | N-[1-(4-Fluoro-phenyl)-ethyl]-N''-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 7 receptor | Bioorg Med Chem Lett 14: 4245-8 (2004) Article DOI: 10.1016/j.bmcl.2004.06.008 BindingDB Entry DOI: 10.7270/Q2GM86RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50150418 (CHEMBL183347 | N-[1-(4-Fluoro-phenyl)-ethyl]-N''-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 7 receptor | Bioorg Med Chem Lett 14: 4245-8 (2004) Article DOI: 10.1016/j.bmcl.2004.06.008 BindingDB Entry DOI: 10.7270/Q2GM86RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477513 (CHEMBL392246) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma secretase assessed as reduction of amyloid beta level in H4 cells | Bioorg Med Chem Lett 17: 4006-11 (2007) Article DOI: 10.1016/j.bmcl.2007.04.082 BindingDB Entry DOI: 10.7270/Q2XD14GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477516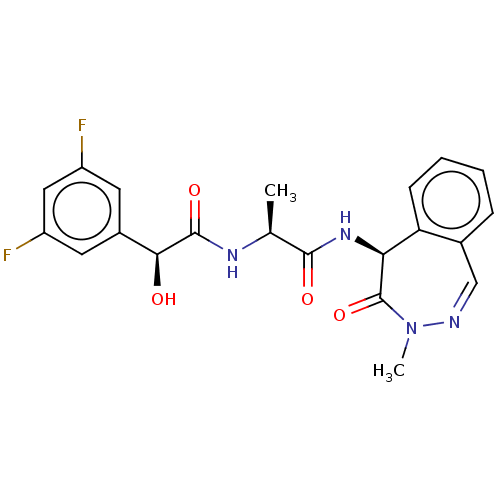 (BMS-433796 | CHEMBL247361) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma secretase assessed as reduction of amyloid beta level in H4 cells | Bioorg Med Chem Lett 17: 4006-11 (2007) Article DOI: 10.1016/j.bmcl.2007.04.082 BindingDB Entry DOI: 10.7270/Q2XD14GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477528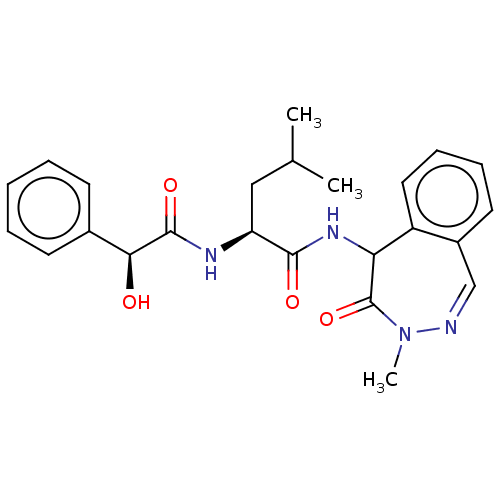 (CHEMBL397844) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma secretase assessed as reduction of amyloid beta level in H4 cells | Bioorg Med Chem Lett 17: 4006-11 (2007) Article DOI: 10.1016/j.bmcl.2007.04.082 BindingDB Entry DOI: 10.7270/Q2XD14GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477531 (CHEMBL396808) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma secretase assessed as reduction of amyloid beta level in H4 cells | Bioorg Med Chem Lett 17: 4006-11 (2007) Article DOI: 10.1016/j.bmcl.2007.04.082 BindingDB Entry DOI: 10.7270/Q2XD14GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477536 (CHEMBL247155) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma secretase assessed as reduction of amyloid beta level in H4 cells | Bioorg Med Chem Lett 17: 4006-11 (2007) Article DOI: 10.1016/j.bmcl.2007.04.082 BindingDB Entry DOI: 10.7270/Q2XD14GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477521 (CHEMBL439210) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma secretase assessed as reduction of amyloid beta level in H4 cells | Bioorg Med Chem Lett 17: 4006-11 (2007) Article DOI: 10.1016/j.bmcl.2007.04.082 BindingDB Entry DOI: 10.7270/Q2XD14GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477526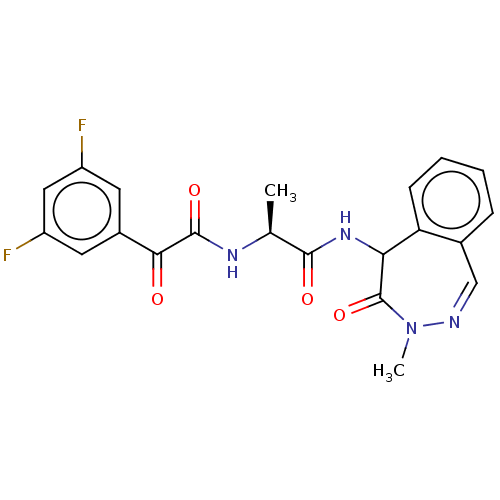 (CHEMBL429292) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma secretase assessed as reduction of amyloid beta level in H4 cells | Bioorg Med Chem Lett 17: 4006-11 (2007) Article DOI: 10.1016/j.bmcl.2007.04.082 BindingDB Entry DOI: 10.7270/Q2XD14GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477517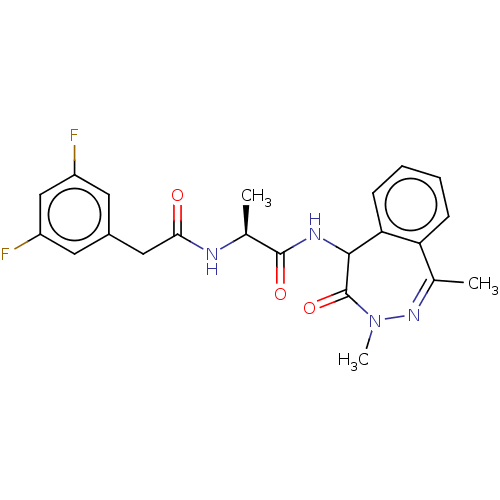 (CHEMBL246785) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma secretase assessed as reduction of amyloid beta level in H4 cells | Bioorg Med Chem Lett 17: 4006-11 (2007) Article DOI: 10.1016/j.bmcl.2007.04.082 BindingDB Entry DOI: 10.7270/Q2XD14GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477534 (CHEMBL397394) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma secretase assessed as reduction of amyloid beta level in H4 cells | Bioorg Med Chem Lett 17: 4006-11 (2007) Article DOI: 10.1016/j.bmcl.2007.04.082 BindingDB Entry DOI: 10.7270/Q2XD14GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477511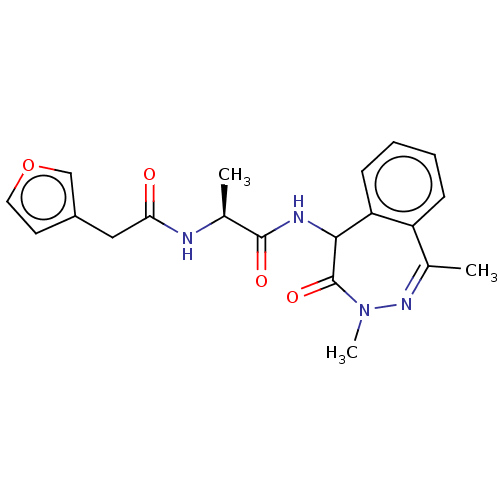 (CHEMBL245385) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma secretase assessed as reduction of amyloid beta level in H4 cells | Bioorg Med Chem Lett 17: 4006-11 (2007) Article DOI: 10.1016/j.bmcl.2007.04.082 BindingDB Entry DOI: 10.7270/Q2XD14GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477519 (CHEMBL397335) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma secretase assessed as reduction of amyloid beta level in H4 cells | Bioorg Med Chem Lett 17: 4006-11 (2007) Article DOI: 10.1016/j.bmcl.2007.04.082 BindingDB Entry DOI: 10.7270/Q2XD14GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477530 (CHEMBL246575) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma secretase assessed as reduction of amyloid beta level in H4 cells | Bioorg Med Chem Lett 17: 4006-11 (2007) Article DOI: 10.1016/j.bmcl.2007.04.082 BindingDB Entry DOI: 10.7270/Q2XD14GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477524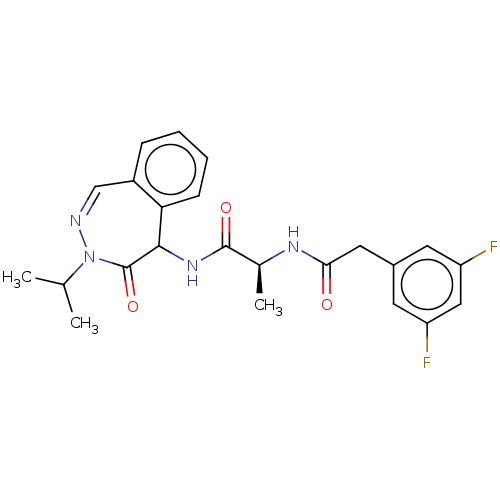 (CHEMBL246784) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma secretase assessed as reduction of amyloid beta level in H4 cells | Bioorg Med Chem Lett 17: 4006-11 (2007) Article DOI: 10.1016/j.bmcl.2007.04.082 BindingDB Entry DOI: 10.7270/Q2XD14GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477533 (CHEMBL246369) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma secretase assessed as reduction of amyloid beta level in H4 cells | Bioorg Med Chem Lett 17: 4006-11 (2007) Article DOI: 10.1016/j.bmcl.2007.04.082 BindingDB Entry DOI: 10.7270/Q2XD14GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477535 (CHEMBL397547) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma secretase assessed as reduction of amyloid beta level in H4 cells | Bioorg Med Chem Lett 17: 4006-11 (2007) Article DOI: 10.1016/j.bmcl.2007.04.082 BindingDB Entry DOI: 10.7270/Q2XD14GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477518 (CHEMBL436997) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma secretase assessed as reduction of amyloid beta level in H4 cells | Bioorg Med Chem Lett 17: 4006-11 (2007) Article DOI: 10.1016/j.bmcl.2007.04.082 BindingDB Entry DOI: 10.7270/Q2XD14GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477525 (CHEMBL247560) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma secretase assessed as reduction of amyloid beta level in H4 cells | Bioorg Med Chem Lett 17: 4006-11 (2007) Article DOI: 10.1016/j.bmcl.2007.04.082 BindingDB Entry DOI: 10.7270/Q2XD14GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477514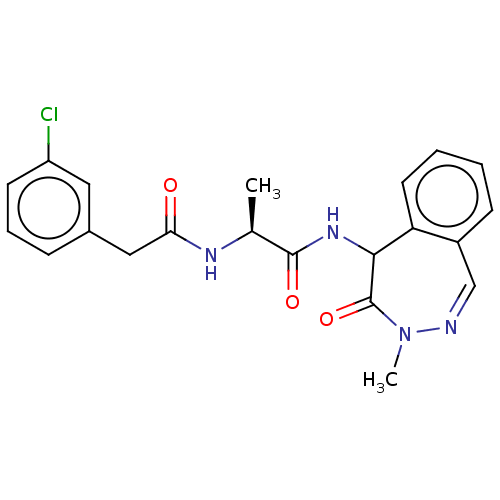 (CHEMBL397548) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma secretase assessed as reduction of amyloid beta level in H4 cells | Bioorg Med Chem Lett 17: 4006-11 (2007) Article DOI: 10.1016/j.bmcl.2007.04.082 BindingDB Entry DOI: 10.7270/Q2XD14GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477537 (CHEMBL247154) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma secretase assessed as reduction of amyloid beta level in H4 cells | Bioorg Med Chem Lett 17: 4006-11 (2007) Article DOI: 10.1016/j.bmcl.2007.04.082 BindingDB Entry DOI: 10.7270/Q2XD14GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477532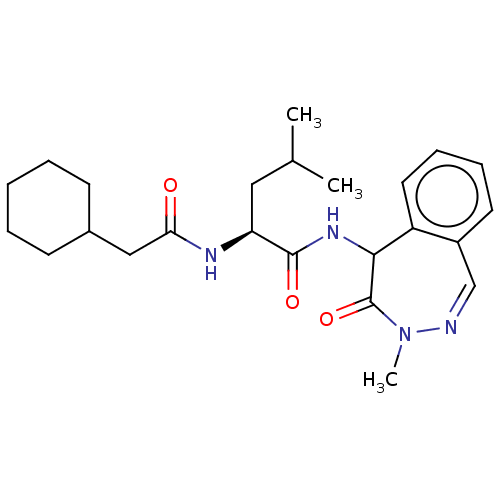 (CHEMBL247360) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma secretase assessed as reduction of amyloid beta level in H4 cells | Bioorg Med Chem Lett 17: 4006-11 (2007) Article DOI: 10.1016/j.bmcl.2007.04.082 BindingDB Entry DOI: 10.7270/Q2XD14GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477527 (CHEMBL396164) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma secretase assessed as reduction of amyloid beta level in H4 cells | Bioorg Med Chem Lett 17: 4006-11 (2007) Article DOI: 10.1016/j.bmcl.2007.04.082 BindingDB Entry DOI: 10.7270/Q2XD14GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477512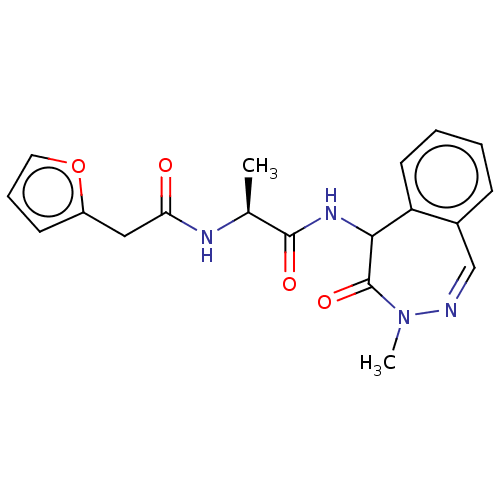 (CHEMBL265897) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma secretase assessed as reduction of amyloid beta level in H4 cells | Bioorg Med Chem Lett 17: 4006-11 (2007) Article DOI: 10.1016/j.bmcl.2007.04.082 BindingDB Entry DOI: 10.7270/Q2XD14GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477538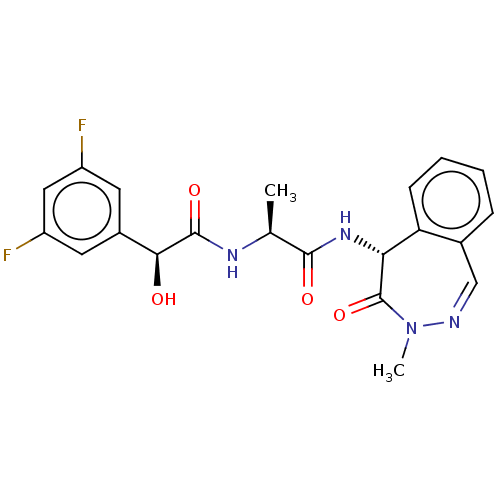 (CHEMBL396639) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma secretase assessed as reduction of amyloid beta level in H4 cells | Bioorg Med Chem Lett 17: 4006-11 (2007) Article DOI: 10.1016/j.bmcl.2007.04.082 BindingDB Entry DOI: 10.7270/Q2XD14GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 111 total ) | Next | Last >> |