

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM12178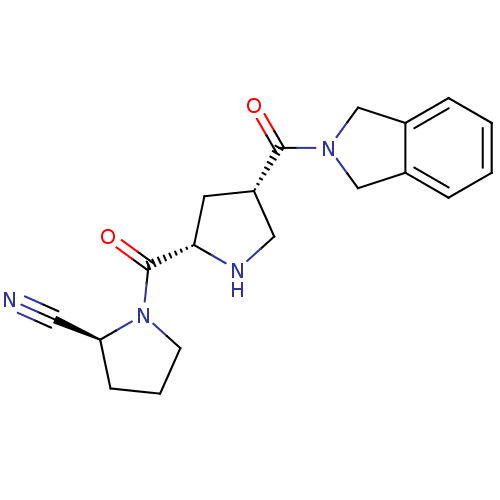 ((2S)-1-{[(2S,4S)-4-(2,3-dihydro-1H-isoindol-2-ylca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11719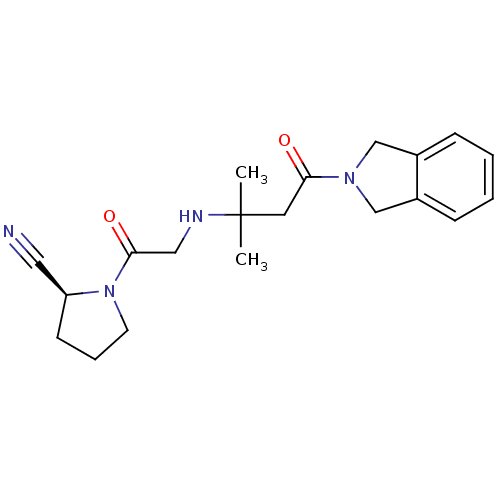 ((2S)-1-(2-{[4-(2,3-dihydro-1H-isoindol-2-yl)-2-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11162 ((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM12167 (5-chloro-2-{[(3S,5S)-5-(pyrrolidin-1-ylcarbonyl)py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11695 ((2S)-1-{2-[(3-hydroxyadamantan-1-yl)amino]acetyl}p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM12169 (5-methyl-2-{[(3S,5S)-5-(pyrrolidin-1-ylcarbonyl)py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM12166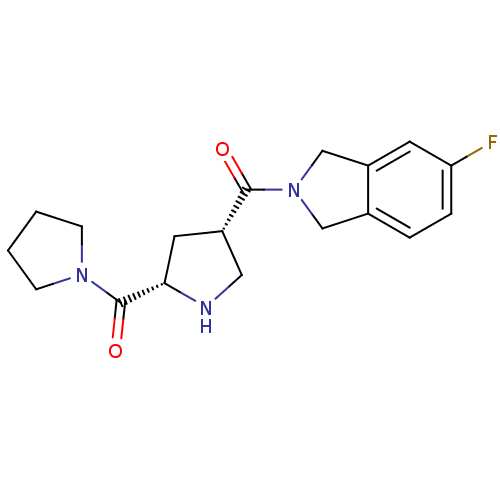 (5-fluoro-2-{[(3S,5S)-5-(pyrrolidin-1-ylcarbonyl)py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM12168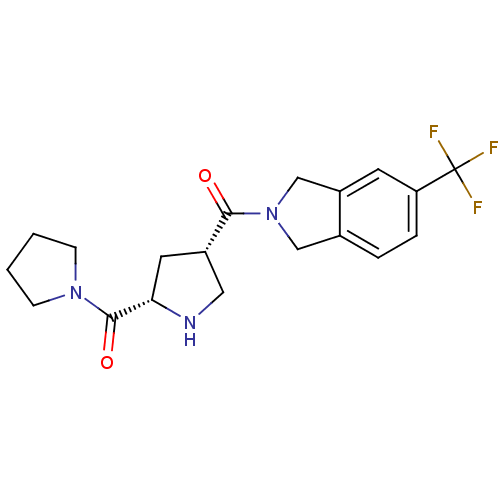 (2-{[(3S,5S)-5-(pyrrolidin-1-ylcarbonyl)pyrrolidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM12174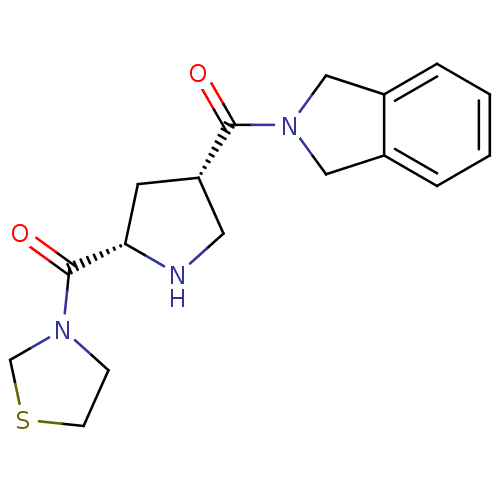 (3-{[(2S,4S)-4-(2,3-dihydro-1H-isoindol-2-ylcarbony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM12165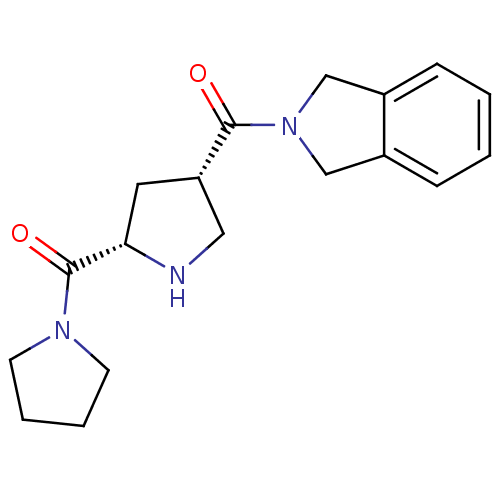 (2-{[(3S,5S)-5-(pyrrolidin-1-ylcarbonyl)pyrrolidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 109 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM12177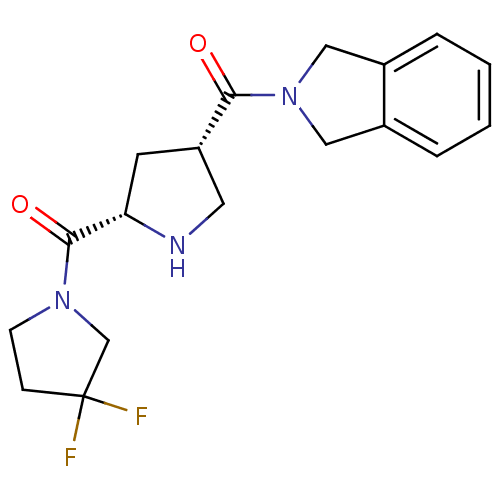 (2-{[(3S,5S)-5-[(3,3-difluoropyrrolidin-1-yl)carbon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 129 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM12170 (5,6-dichloro-2-{[(3S,5S)-5-(pyrrolidin-1-ylcarbony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 144 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Homo sapiens (Human)) | BDBM12178 ((2S)-1-{[(2S,4S)-4-(2,3-dihydro-1H-isoindol-2-ylca...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 175 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM12176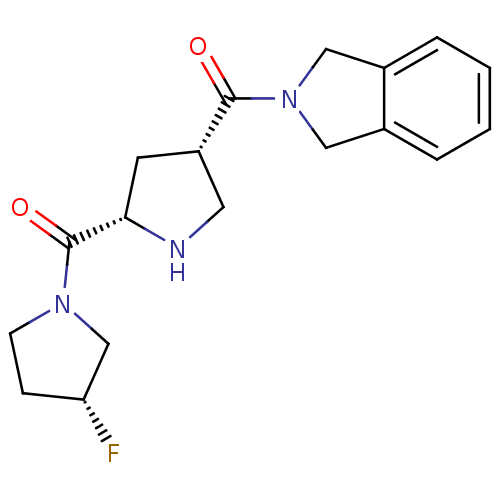 (2-{[(3S,5S)-5-{[(3R)-3-fluoropyrrolidin-1-yl]carbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 228 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM12175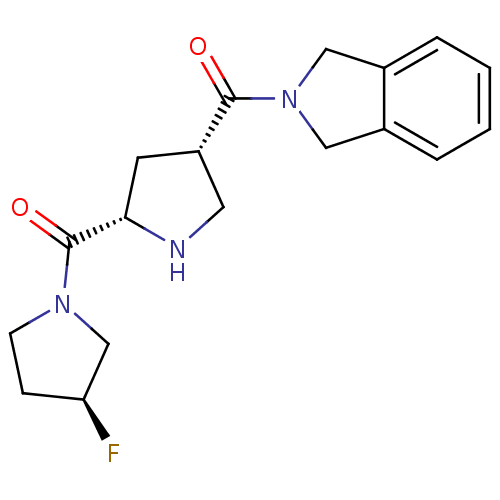 (2-{[(3S,5S)-5-{[(3S)-3-fluoropyrrolidin-1-yl]carbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 245 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM12179 ((2S)-1-{[(2S,4S)-4-(2,3-dihydro-1H-isoindol-2-ylca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 984 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM12172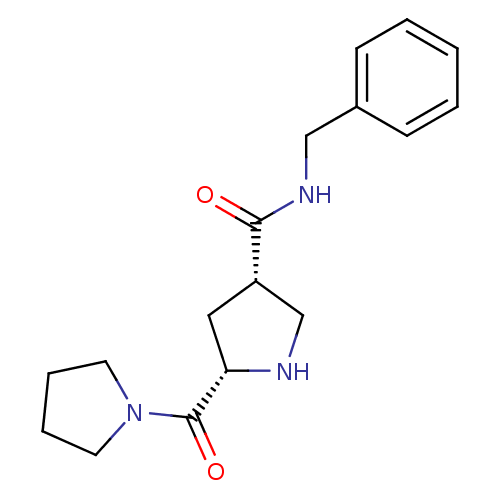 ((3S,5S)-N-benzyl-5-(pyrrolidin-1-ylcarbonyl)pyrrol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.96E+3 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM12173 ((3S,5S)-N-(2-phenylethyl)-5-(pyrrolidin-1-ylcarbon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.59E+3 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM12178 ((2S)-1-{[(2S,4S)-4-(2,3-dihydro-1H-isoindol-2-ylca...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM12171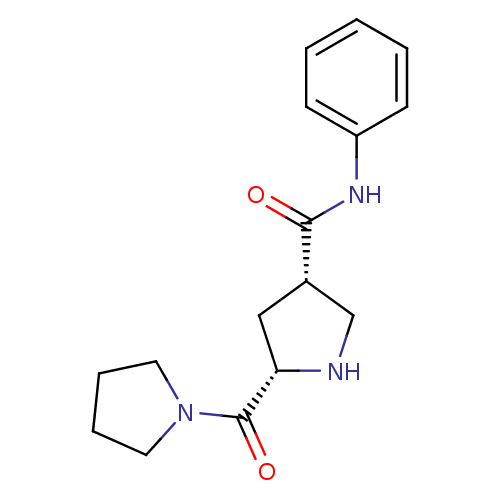 ((3S,5S)-N-phenyl-5-(pyrrolidin-1-ylcarbonyl)pyrrol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.13E+3 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Homo sapiens (Human)) | BDBM12170 (5,6-dichloro-2-{[(3S,5S)-5-(pyrrolidin-1-ylcarbony...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Homo sapiens (Human)) | BDBM12167 (5-chloro-2-{[(3S,5S)-5-(pyrrolidin-1-ylcarbonyl)py...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Homo sapiens (Human)) | BDBM12169 (5-methyl-2-{[(3S,5S)-5-(pyrrolidin-1-ylcarbonyl)py...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Homo sapiens (Human)) | BDBM12174 (3-{[(2S,4S)-4-(2,3-dihydro-1H-isoindol-2-ylcarbony...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM12170 (5,6-dichloro-2-{[(3S,5S)-5-(pyrrolidin-1-ylcarbony...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Homo sapiens (Human)) | BDBM12166 (5-fluoro-2-{[(3S,5S)-5-(pyrrolidin-1-ylcarbonyl)py...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM11695 ((2S)-1-{2-[(3-hydroxyadamantan-1-yl)amino]acetyl}p...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Homo sapiens (Human)) | BDBM12165 (2-{[(3S,5S)-5-(pyrrolidin-1-ylcarbonyl)pyrrolidin-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM12177 (2-{[(3S,5S)-5-[(3,3-difluoropyrrolidin-1-yl)carbon...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Homo sapiens (Human)) | BDBM12177 (2-{[(3S,5S)-5-[(3,3-difluoropyrrolidin-1-yl)carbon...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM12175 (2-{[(3S,5S)-5-{[(3S)-3-fluoropyrrolidin-1-yl]carbo...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM12176 (2-{[(3S,5S)-5-{[(3R)-3-fluoropyrrolidin-1-yl]carbo...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM12168 (2-{[(3S,5S)-5-(pyrrolidin-1-ylcarbonyl)pyrrolidin-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM11695 ((2S)-1-{2-[(3-hydroxyadamantan-1-yl)amino]acetyl}p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Homo sapiens (Human)) | BDBM11719 ((2S)-1-(2-{[4-(2,3-dihydro-1H-isoindol-2-yl)-2-met...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM11162 ((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM12165 (2-{[(3S,5S)-5-(pyrrolidin-1-ylcarbonyl)pyrrolidin-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM12175 (2-{[(3S,5S)-5-{[(3S)-3-fluoropyrrolidin-1-yl]carbo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Homo sapiens (Human)) | BDBM12168 (2-{[(3S,5S)-5-(pyrrolidin-1-ylcarbonyl)pyrrolidin-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM12167 (5-chloro-2-{[(3S,5S)-5-(pyrrolidin-1-ylcarbonyl)py...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM11719 ((2S)-1-(2-{[4-(2,3-dihydro-1H-isoindol-2-yl)-2-met...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM11162 ((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM12165 (2-{[(3S,5S)-5-(pyrrolidin-1-ylcarbonyl)pyrrolidin-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM12172 ((3S,5S)-N-benzyl-5-(pyrrolidin-1-ylcarbonyl)pyrrol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM12174 (3-{[(2S,4S)-4-(2,3-dihydro-1H-isoindol-2-ylcarbony...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM12179 ((2S)-1-{[(2S,4S)-4-(2,3-dihydro-1H-isoindol-2-ylca...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Homo sapiens (Human)) | BDBM12172 ((3S,5S)-N-benzyl-5-(pyrrolidin-1-ylcarbonyl)pyrrol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM12166 (5-fluoro-2-{[(3S,5S)-5-(pyrrolidin-1-ylcarbonyl)py...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM12168 (2-{[(3S,5S)-5-(pyrrolidin-1-ylcarbonyl)pyrrolidin-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM12169 (5-methyl-2-{[(3S,5S)-5-(pyrrolidin-1-ylcarbonyl)py...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM12171 ((3S,5S)-N-phenyl-5-(pyrrolidin-1-ylcarbonyl)pyrrol...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM12167 (5-chloro-2-{[(3S,5S)-5-(pyrrolidin-1-ylcarbonyl)py...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM12169 (5-methyl-2-{[(3S,5S)-5-(pyrrolidin-1-ylcarbonyl)py...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM12177 (2-{[(3S,5S)-5-[(3,3-difluoropyrrolidin-1-yl)carbon...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Homo sapiens (Human)) | BDBM12171 ((3S,5S)-N-phenyl-5-(pyrrolidin-1-ylcarbonyl)pyrrol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Homo sapiens (Human)) | BDBM12179 ((2S)-1-{[(2S,4S)-4-(2,3-dihydro-1H-isoindol-2-ylca...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Homo sapiens (Human)) | BDBM11162 ((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM12172 ((3S,5S)-N-benzyl-5-(pyrrolidin-1-ylcarbonyl)pyrrol...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM12173 ((3S,5S)-N-(2-phenylethyl)-5-(pyrrolidin-1-ylcarbon...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM12179 ((2S)-1-{[(2S,4S)-4-(2,3-dihydro-1H-isoindol-2-ylca...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Homo sapiens (Human)) | BDBM12173 ((3S,5S)-N-(2-phenylethyl)-5-(pyrrolidin-1-ylcarbon...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Homo sapiens (Human)) | BDBM12175 (2-{[(3S,5S)-5-{[(3S)-3-fluoropyrrolidin-1-yl]carbo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Homo sapiens (Human)) | BDBM11695 ((2S)-1-{2-[(3-hydroxyadamantan-1-yl)amino]acetyl}p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM12174 (3-{[(2S,4S)-4-(2,3-dihydro-1H-isoindol-2-ylcarbony...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM12166 (5-fluoro-2-{[(3S,5S)-5-(pyrrolidin-1-ylcarbonyl)py...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM12170 (5,6-dichloro-2-{[(3S,5S)-5-(pyrrolidin-1-ylcarbony...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM12171 ((3S,5S)-N-phenyl-5-(pyrrolidin-1-ylcarbonyl)pyrrol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM12173 ((3S,5S)-N-(2-phenylethyl)-5-(pyrrolidin-1-ylcarbon...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM12176 (2-{[(3S,5S)-5-{[(3R)-3-fluoropyrrolidin-1-yl]carbo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM12178 ((2S)-1-{[(2S,4S)-4-(2,3-dihydro-1H-isoindol-2-ylca...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM11719 ((2S)-1-(2-{[4-(2,3-dihydro-1H-isoindol-2-yl)-2-met...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Homo sapiens (Human)) | BDBM12176 (2-{[(3S,5S)-5-{[(3R)-3-fluoropyrrolidin-1-yl]carbo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||