

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10597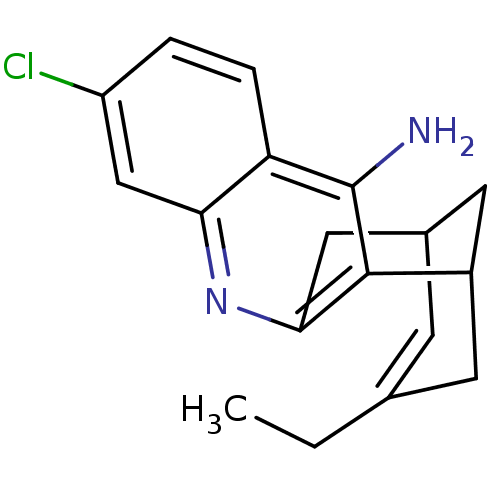 ((1S)-7-chloro-15-ethyl-10-azatetracyclo[11.3.1.0^{...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0260 | -60.4 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description The cholinesterase assays were performed using colorimetric method reported by E llman. Inhibition of enzyme activity was measured over a substrate c... | J Med Chem 49: 3421-5 (2006) Article DOI: 10.1021/jm060257t BindingDB Entry DOI: 10.7270/Q2WW7FWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10595 ((9E)-7-(7-(1,2,3,4-Tetrahydroacridin-9-ylamino)hep...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.40 | -46.8 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description The cholinesterase assays were performed using colorimetric method reported by E llman. Inhibition of enzyme activity was measured over a substrate c... | J Med Chem 49: 3421-5 (2006) Article DOI: 10.1021/jm060257t BindingDB Entry DOI: 10.7270/Q2WW7FWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 7 | -46.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description The cholinesterase assays were performed using colorimetric method reported by E llman. Inhibition of enzyme activity was measured over a substrate c... | J Med Chem 49: 3421-5 (2006) Article DOI: 10.1021/jm060257t BindingDB Entry DOI: 10.7270/Q2WW7FWB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10594 ((9E)-N1-(7-(1,2,3,4-Tetrahydroacridin-9-ylamino)he...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 15.7 | -44.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description The cholinesterase assays were performed using colorimetric method reported by E llman. Inhibition of enzyme activity was measured over a substrate c... | J Med Chem 49: 3421-5 (2006) Article DOI: 10.1021/jm060257t BindingDB Entry DOI: 10.7270/Q2WW7FWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10593 ((9E)-N1-(7-(1,2,3,4-Tetrahydroacridin-9-ylamino)he...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 16.5 | -44.4 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description The cholinesterase assays were performed using colorimetric method reported by E llman. Inhibition of enzyme activity was measured over a substrate c... | J Med Chem 49: 3421-5 (2006) Article DOI: 10.1021/jm060257t BindingDB Entry DOI: 10.7270/Q2WW7FWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10595 ((9E)-7-(7-(1,2,3,4-Tetrahydroacridin-9-ylamino)hep...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 19.5 | -44.0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description The cholinesterase assays were performed using colorimetric method reported by E llman. Inhibition of enzyme activity was measured over a substrate c... | J Med Chem 49: 3421-5 (2006) Article DOI: 10.1021/jm060257t BindingDB Entry DOI: 10.7270/Q2WW7FWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10593 ((9E)-N1-(7-(1,2,3,4-Tetrahydroacridin-9-ylamino)he...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20.8 | -43.8 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description The cholinesterase assays were performed using colorimetric method reported by E llman. Inhibition of enzyme activity was measured over a substrate c... | J Med Chem 49: 3421-5 (2006) Article DOI: 10.1021/jm060257t BindingDB Entry DOI: 10.7270/Q2WW7FWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10594 ((9E)-N1-(7-(1,2,3,4-Tetrahydroacridin-9-ylamino)he...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 30.8 | -42.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description The cholinesterase assays were performed using colorimetric method reported by E llman. Inhibition of enzyme activity was measured over a substrate c... | J Med Chem 49: 3421-5 (2006) Article DOI: 10.1021/jm060257t BindingDB Entry DOI: 10.7270/Q2WW7FWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10441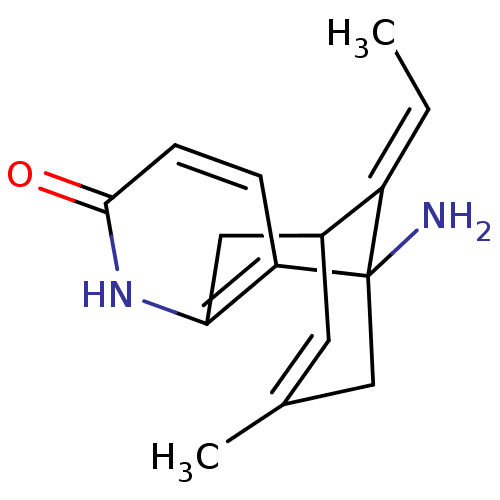 ((+)-Huperzine A | (+/-)Huperzine A | (-)-Huperzine...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 47 | -41.8 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description The cholinesterase assays were performed using colorimetric method reported by E llman. Inhibition of enzyme activity was measured over a substrate c... | J Med Chem 49: 3421-5 (2006) Article DOI: 10.1021/jm060257t BindingDB Entry DOI: 10.7270/Q2WW7FWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10597 ((1S)-7-chloro-15-ethyl-10-azatetracyclo[11.3.1.0^{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 120 | -39.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description The cholinesterase assays were performed using colorimetric method reported by E llman. Inhibition of enzyme activity was measured over a substrate c... | J Med Chem 49: 3421-5 (2006) Article DOI: 10.1021/jm060257t BindingDB Entry DOI: 10.7270/Q2WW7FWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 137 | -39.2 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description The cholinesterase assays were performed using colorimetric method reported by E llman. Inhibition of enzyme activity was measured over a substrate c... | J Med Chem 49: 3421-5 (2006) Article DOI: 10.1021/jm060257t BindingDB Entry DOI: 10.7270/Q2WW7FWB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10441 ((+)-Huperzine A | (+/-)Huperzine A | (-)-Huperzine...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | >1.00E+3 | >-34.2 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description The cholinesterase assays were performed using colorimetric method reported by E llman. Inhibition of enzyme activity was measured over a substrate c... | J Med Chem 49: 3421-5 (2006) Article DOI: 10.1021/jm060257t BindingDB Entry DOI: 10.7270/Q2WW7FWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||