

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50199173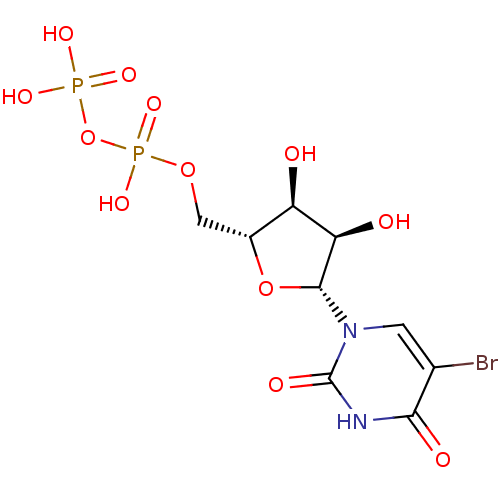 (((2R,3S,4R,5R)-5-(5-bromo-2,4-dioxo-3,4-dihydropyr...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 151 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y6 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50199184 (5-BROMO-URIDINE-5'-MONOPHOSPHATE | 5-bromo-1-beta-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y6 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50199189 (6-methyl-3-beta-D-ribofuranosyl(1H)pyrimidine-2,4-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y6 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 4 (Homo sapiens (Human)) | BDBM50199190 (3-METHYLURIDINE-5'-MONOPHOSHATE | 3-methyl-1-beta-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y4 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50199180 (1-(BETA-D-RIBOFURANOSYL)-2-THIO-URACIL-5'-PHOSPHAT...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y6 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 4 (Homo sapiens (Human)) | BDBM50199182 (6-propyl-3-beta-D-ribofuranosyl(1H)pyrimidine-2,4-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y4 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 4 (Homo sapiens (Human)) | BDBM50118213 (5'-UTP | CHEMBL336296 | H4utp | UTP | uridine 5'-(...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 87 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y4 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50199176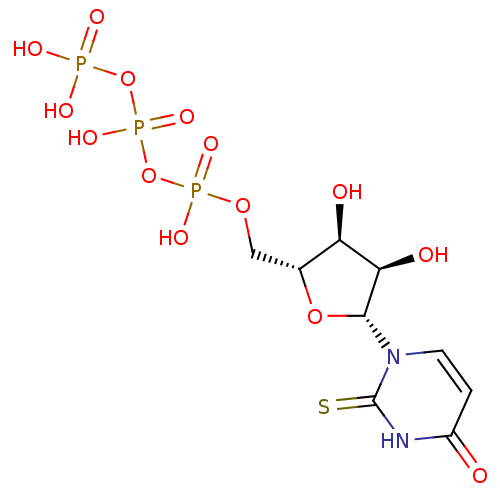 (2-thio-1-beta-D-ribofuranosyl(3H)pyrimidine-2,4-di...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y6 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50199182 (6-propyl-3-beta-D-ribofuranosyl(1H)pyrimidine-2,4-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y6 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50199179 (6-methyl-3-beta-D-ribofuranosyl(1H)pyrimidine-2,4-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y2 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 4 (Homo sapiens (Human)) | BDBM50199183 (3-methyl-1-beta-D-ribofuranosylpyrimidine-2,4-dion...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.92E+3 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y4 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50199179 (6-methyl-3-beta-D-ribofuranosyl(1H)pyrimidine-2,4-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y6 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 4 (Homo sapiens (Human)) | BDBM50199189 (6-methyl-3-beta-D-ribofuranosyl(1H)pyrimidine-2,4-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y4 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50199187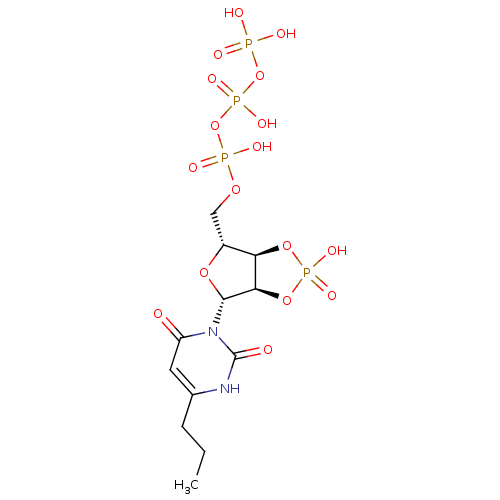 (6-propyl-3-beta-D-ribofuranosyl(1H)pyrimidine-2,4-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y2 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 4 (Homo sapiens (Human)) | BDBM50194162 (3-methyl-1-beta-D-ribofuranosylpyrimidine-2,4-dion...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y4 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50199187 (6-propyl-3-beta-D-ribofuranosyl(1H)pyrimidine-2,4-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y6 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50199193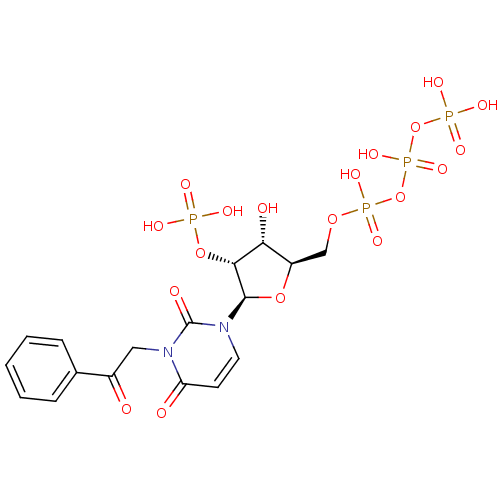 (3-phenacyl-1-beta-D-ribofuranosylpyrimidine-2,4-di...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y6 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50199188 (1-beta-D-ribofuranosyl(3H)pyrimidine-2,4,6-trione ...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y6 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50199174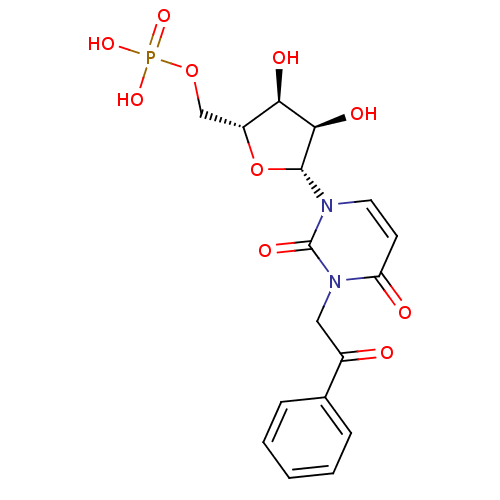 (3-phenacyl-1-beta-D-ribofuranosylpyrimidine-2,4-di...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y2 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50199175 (6-methyl-3-beta-D-ribofuranosyl(1H)pyrimidine-2,4-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y6 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50199174 (3-phenacyl-1-beta-D-ribofuranosylpyrimidine-2,4-di...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y6 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 4 (Homo sapiens (Human)) | BDBM50199180 (1-(BETA-D-RIBOFURANOSYL)-2-THIO-URACIL-5'-PHOSPHAT...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y4 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 4 (Homo sapiens (Human)) | BDBM50199178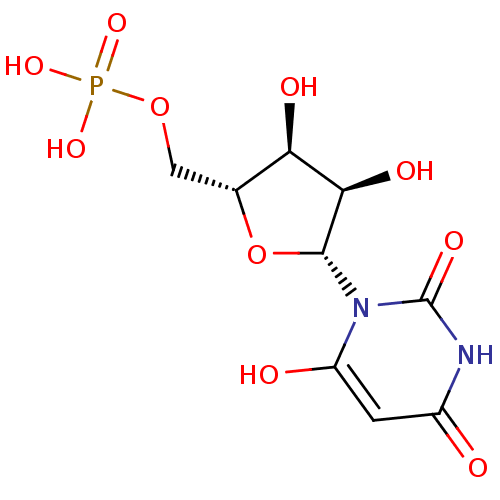 (1-beta-D-ribofuranosyl(3H)pyrimidine-2,4,6-trione ...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y4 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50199188 (1-beta-D-ribofuranosyl(3H)pyrimidine-2,4,6-trione ...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y2 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 4 (Homo sapiens (Human)) | BDBM50199186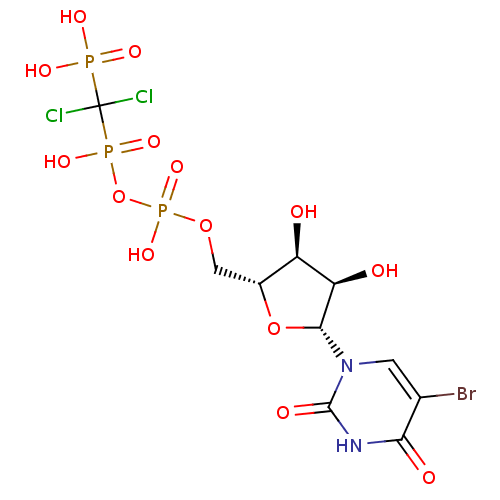 (5-bromouridine-5'-uridylic acid (1,1-dichloro-1-ph...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.99E+3 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y4 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50199190 (3-METHYLURIDINE-5'-MONOPHOSHATE | 3-methyl-1-beta-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y2 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50194162 (3-methyl-1-beta-D-ribofuranosylpyrimidine-2,4-dion...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y2 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 4 (Homo sapiens (Human)) | BDBM50199181 (6-propyl-3-beta-D-ribofuranosyl(1H)pyrimidine-2,4-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y4 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50199172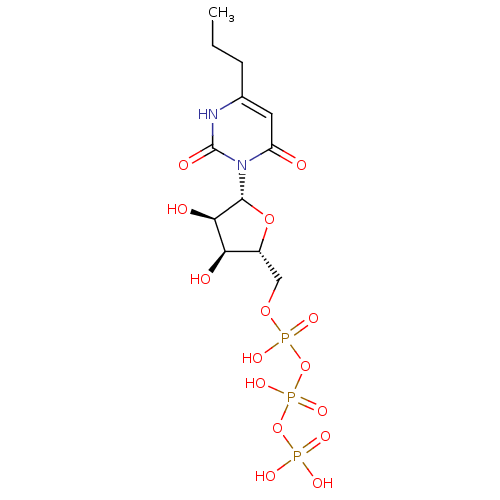 (6-propyl-3-beta-D-ribofuranosyl(1H)pyrimidine-2,4-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y6 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50118239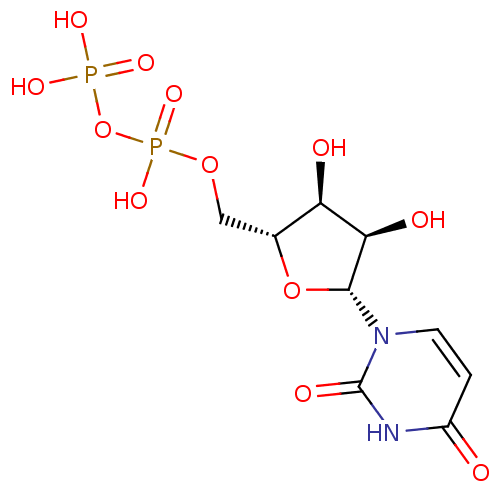 (CHEMBL130266 | UDP | Uridine diphosphate | uridine...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 42 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y6 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50199178 (1-beta-D-ribofuranosyl(3H)pyrimidine-2,4,6-trione ...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y2 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50199192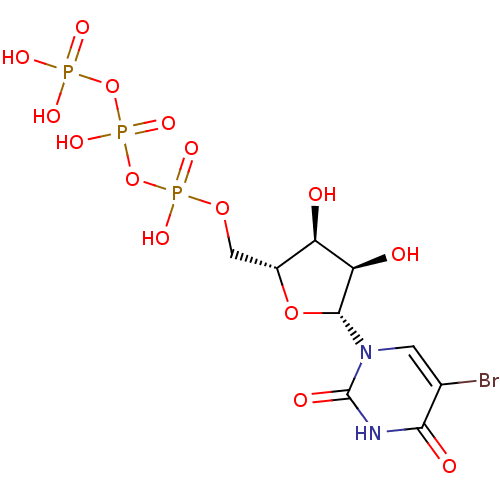 (((2R,3S,4R,5R)-5-(5-bromo-2,4-dioxo-3,4-dihydropyr...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 291 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y6 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50199177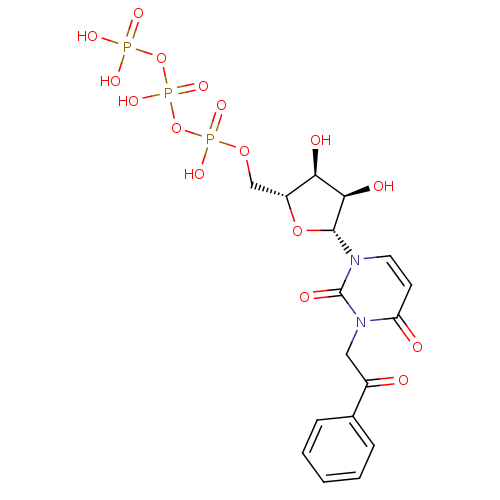 (3-phenacyl-1-beta-D-ribofuranosylpyrimidine-2,4-di...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y2 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50199184 (5-BROMO-URIDINE-5'-MONOPHOSPHATE | 5-bromo-1-beta-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y2 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 4 (Homo sapiens (Human)) | BDBM50199174 (3-phenacyl-1-beta-D-ribofuranosylpyrimidine-2,4-di...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y4 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 4 (Homo sapiens (Human)) | BDBM50199176 (2-thio-1-beta-D-ribofuranosyl(3H)pyrimidine-2,4-di...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.77E+3 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y4 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Rattus norvegicus) | BDBM50199173 (((2R,3S,4R,5R)-5-(5-bromo-2,4-dioxo-3,4-dihydropyr...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at rat P2Y6 receptor assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50199176 (2-thio-1-beta-D-ribofuranosyl(3H)pyrimidine-2,4-di...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y2 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 4 (Homo sapiens (Human)) | BDBM50199187 (6-propyl-3-beta-D-ribofuranosyl(1H)pyrimidine-2,4-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y4 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 4 (Homo sapiens (Human)) | BDBM50199177 (3-phenacyl-1-beta-D-ribofuranosylpyrimidine-2,4-di...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y4 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50199175 (6-methyl-3-beta-D-ribofuranosyl(1H)pyrimidine-2,4-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y2 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50194162 (3-methyl-1-beta-D-ribofuranosylpyrimidine-2,4-dion...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y6 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50199186 (5-bromouridine-5'-uridylic acid (1,1-dichloro-1-ph...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 354 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y2 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50199182 (6-propyl-3-beta-D-ribofuranosyl(1H)pyrimidine-2,4-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y2 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 4 (Homo sapiens (Human)) | BDBM50199191 (((2R,3S,4R,5R)-5-(2,4-dioxo-3-(2-oxo-2-phenylethyl...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y4 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50199189 (6-methyl-3-beta-D-ribofuranosyl(1H)pyrimidine-2,4-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y2 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50194160 ((2R,3R,4S,5R)-1-(3,4-dihydroxy-5-(diphosphoryloxym...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y2 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50199186 (5-bromouridine-5'-uridylic acid (1,1-dichloro-1-ph...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 120 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y6 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50199183 (3-methyl-1-beta-D-ribofuranosylpyrimidine-2,4-dion...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 564 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y2 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50199183 (3-methyl-1-beta-D-ribofuranosylpyrimidine-2,4-dion...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y6 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 4 (Homo sapiens (Human)) | BDBM50199184 (5-BROMO-URIDINE-5'-MONOPHOSPHATE | 5-bromo-1-beta-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y4 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 4 (Homo sapiens (Human)) | BDBM50194160 ((2R,3R,4S,5R)-1-(3,4-dihydroxy-5-(diphosphoryloxym...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y4 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50199172 (6-propyl-3-beta-D-ribofuranosyl(1H)pyrimidine-2,4-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y2 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 4 (Homo sapiens (Human)) | BDBM50199193 (3-phenacyl-1-beta-D-ribofuranosylpyrimidine-2,4-di...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y4 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 4 (Homo sapiens (Human)) | BDBM50199173 (((2R,3S,4R,5R)-5-(5-bromo-2,4-dioxo-3,4-dihydropyr...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y4 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50199181 (6-propyl-3-beta-D-ribofuranosyl(1H)pyrimidine-2,4-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y2 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 4 (Homo sapiens (Human)) | BDBM50199175 (6-methyl-3-beta-D-ribofuranosyl(1H)pyrimidine-2,4-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y4 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 4 (Homo sapiens (Human)) | BDBM50199188 (1-beta-D-ribofuranosyl(3H)pyrimidine-2,4,6-trione ...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y4 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 4 (Homo sapiens (Human)) | BDBM50199192 (((2R,3S,4R,5R)-5-(5-bromo-2,4-dioxo-3,4-dihydropyr...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.46E+3 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y4 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50199191 (((2R,3S,4R,5R)-5-(2,4-dioxo-3-(2-oxo-2-phenylethyl...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 70 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y6 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50199173 (((2R,3S,4R,5R)-5-(5-bromo-2,4-dioxo-3,4-dihydropyr...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.67E+3 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y2 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50199177 (3-phenacyl-1-beta-D-ribofuranosylpyrimidine-2,4-di...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y6 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50199181 (6-propyl-3-beta-D-ribofuranosyl(1H)pyrimidine-2,4-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y6 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50199178 (1-beta-D-ribofuranosyl(3H)pyrimidine-2,4,6-trione ...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y6 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 4 (Homo sapiens (Human)) | BDBM50199172 (6-propyl-3-beta-D-ribofuranosyl(1H)pyrimidine-2,4-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y4 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50199191 (((2R,3S,4R,5R)-5-(2,4-dioxo-3-(2-oxo-2-phenylethyl...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y2 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50199192 (((2R,3S,4R,5R)-5-(5-bromo-2,4-dioxo-3,4-dihydropyr...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 347 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y2 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 4 (Homo sapiens (Human)) | BDBM50199179 (6-methyl-3-beta-D-ribofuranosyl(1H)pyrimidine-2,4-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y4 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50118213 (5'-UTP | CHEMBL336296 | H4utp | UTP | uridine 5'-(...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 43 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y2 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||