 Found 24 hits of Enzyme Inhibition Constant Data
Found 24 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aromatase
(Homo sapiens (Human)) | BDBM9460

(3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...)Show InChI InChI=1S/C13H16N2O2/c1-2-13(8-7-11(16)15-12(13)17)9-3-5-10(14)6-4-9/h3-6H,2,7-8,14H2,1H3,(H,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human aromatase |
J Med Chem 50: 2799-806 (2007)
Article DOI: 10.1021/jm070109i
BindingDB Entry DOI: 10.7270/Q2668F0Z |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50213235
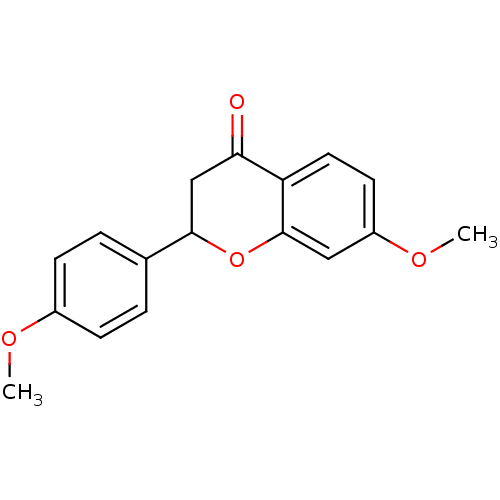
((+/-)-7-methoxy-2-(4-methoxyphenyl)chroman-4-one |...)Show InChI InChI=1S/C17H16O4/c1-19-12-5-3-11(4-6-12)16-10-15(18)14-8-7-13(20-2)9-17(14)21-16/h3-9,16H,10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human aromatase |
J Med Chem 50: 2799-806 (2007)
Article DOI: 10.1021/jm070109i
BindingDB Entry DOI: 10.7270/Q2668F0Z |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50213245
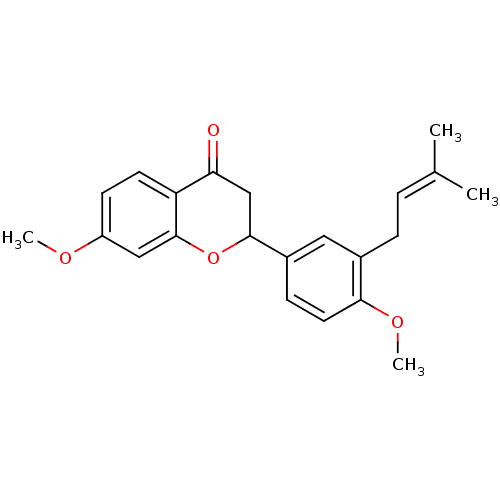
(7-methoxy-2-[4-methoxy-3-(3-methylbut-2-enyl)pheny...)Show SMILES [#6]-[#8]-c1ccc2-[#6](=O)-[#6]-[#6](-[#8]-c2c1)-c1ccc(-[#8]-[#6])c(-[#6]\[#6]=[#6](\[#6])-[#6])c1 Show InChI InChI=1S/C22H24O4/c1-14(2)5-6-15-11-16(7-10-20(15)25-4)21-13-19(23)18-9-8-17(24-3)12-22(18)26-21/h5,7-12,21H,6,13H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human aromatase |
J Med Chem 50: 2799-806 (2007)
Article DOI: 10.1021/jm070109i
BindingDB Entry DOI: 10.7270/Q2668F0Z |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50213239
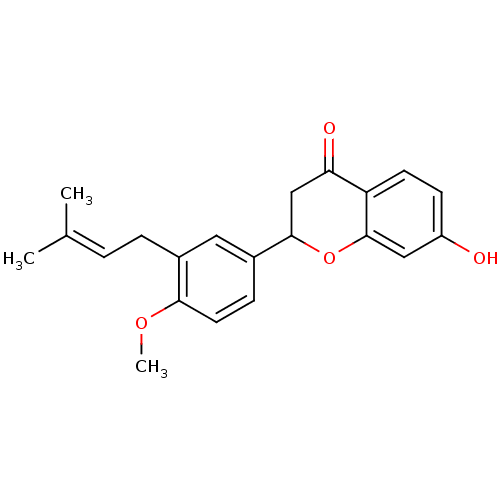
(7-Hydroxy-2-[4'-methoxy-3'-(3-methylbut-2-enyl)phe...)Show SMILES [#6]-[#8]-c1ccc(cc1-[#6]\[#6]=[#6](\[#6])-[#6])-[#6]-1-[#6]-[#6](=O)-c2ccc(-[#8])cc2-[#8]-1 Show InChI InChI=1S/C21H22O4/c1-13(2)4-5-14-10-15(6-9-19(14)24-3)20-12-18(23)17-8-7-16(22)11-21(17)25-20/h4,6-11,20,22H,5,12H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human aromatase |
J Med Chem 50: 2799-806 (2007)
Article DOI: 10.1021/jm070109i
BindingDB Entry DOI: 10.7270/Q2668F0Z |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50213241
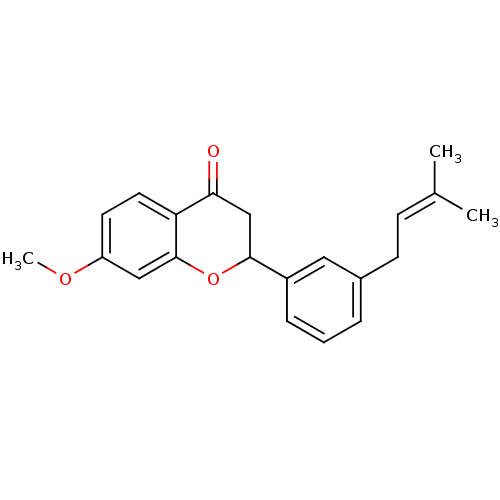
(7-methoxy-2-[3-(3-methylbut-2-enyl)phenyl]chroman-...)Show SMILES [#6]-[#8]-c1ccc2-[#6](=O)-[#6]-[#6](-[#8]-c2c1)-c1cccc(-[#6]\[#6]=[#6](\[#6])-[#6])c1 Show InChI InChI=1S/C21H22O3/c1-14(2)7-8-15-5-4-6-16(11-15)20-13-19(22)18-10-9-17(23-3)12-21(18)24-20/h4-7,9-12,20H,8,13H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human aromatase |
J Med Chem 50: 2799-806 (2007)
Article DOI: 10.1021/jm070109i
BindingDB Entry DOI: 10.7270/Q2668F0Z |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50213236
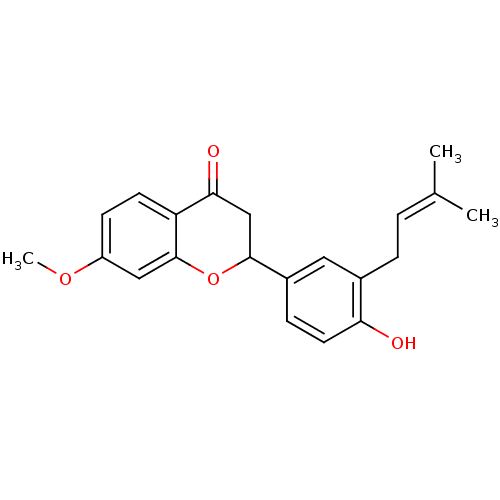
(2-[4-hydroxy-3-(3-methylbut-2-enyl)phenyl]-7-metho...)Show SMILES [#6]-[#8]-c1ccc2-[#6](=O)-[#6]-[#6](-[#8]-c2c1)-c1ccc(-[#8])c(-[#6]\[#6]=[#6](\[#6])-[#6])c1 Show InChI InChI=1S/C21H22O4/c1-13(2)4-5-14-10-15(6-9-18(14)22)20-12-19(23)17-8-7-16(24-3)11-21(17)25-20/h4,6-11,20,22H,5,12H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human aromatase |
J Med Chem 50: 2799-806 (2007)
Article DOI: 10.1021/jm070109i
BindingDB Entry DOI: 10.7270/Q2668F0Z |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50213252
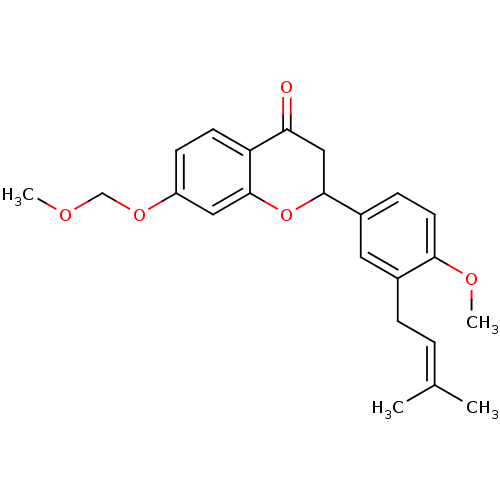
(2-[4-methoxy-3-(3-methylbut-2-enyl)phenyl]-7-(meth...)Show SMILES [#6]-[#8]-[#6]-[#8]-c1ccc2-[#6](=O)-[#6]-[#6](-[#8]-c2c1)-c1ccc(-[#8]-[#6])c(-[#6]\[#6]=[#6](\[#6])-[#6])c1 Show InChI InChI=1S/C23H26O5/c1-15(2)5-6-16-11-17(7-10-21(16)26-4)22-13-20(24)19-9-8-18(27-14-25-3)12-23(19)28-22/h5,7-12,22H,6,13-14H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human aromatase |
J Med Chem 50: 2799-806 (2007)
Article DOI: 10.1021/jm070109i
BindingDB Entry DOI: 10.7270/Q2668F0Z |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50370985
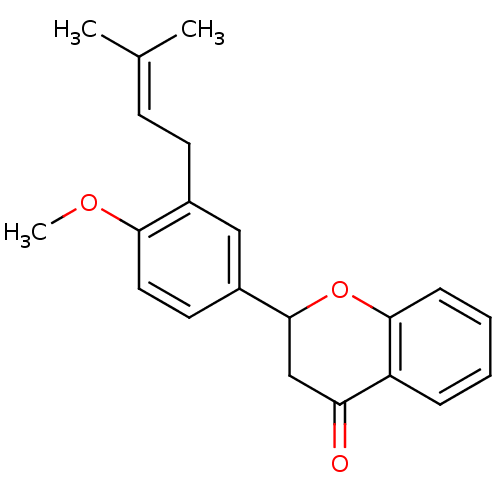
(CHEMBL1162951)Show SMILES [#6]-[#8]-c1ccc(cc1-[#6]\[#6]=[#6](\[#6])-[#6])-[#6]-1-[#6]-[#6](=O)-c2ccccc2-[#8]-1 Show InChI InChI=1S/C21H22O3/c1-14(2)8-9-15-12-16(10-11-19(15)23-3)21-13-18(22)17-6-4-5-7-20(17)24-21/h4-8,10-12,21H,9,13H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human aromatase |
J Med Chem 50: 2799-806 (2007)
Article DOI: 10.1021/jm070109i
BindingDB Entry DOI: 10.7270/Q2668F0Z |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50213234

(7-(methoxymethoxy)-2-[4-(methoxymethoxy)-3-(3-meth...)Show SMILES [#6]-[#8]-[#6]-[#8]-c1ccc2-[#6](=O)-[#6]-[#6](-[#8]-c2c1)-c1ccc(-[#8]-[#6]-[#8]-[#6])c(-[#6]\[#6]=[#6](\[#6])-[#6])c1 Show InChI InChI=1S/C24H28O6/c1-16(2)5-6-17-11-18(7-10-22(17)29-15-27-4)23-13-21(25)20-9-8-19(28-14-26-3)12-24(20)30-23/h5,7-12,23H,6,13-15H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human aromatase |
J Med Chem 50: 2799-806 (2007)
Article DOI: 10.1021/jm070109i
BindingDB Entry DOI: 10.7270/Q2668F0Z |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50213243
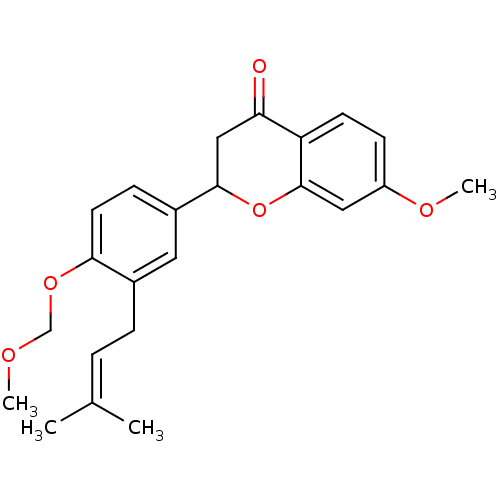
(7-methoxy-2-[4-(methoxymethoxy)-3-(3-methylbut-2-e...)Show SMILES [#6]-[#8]-[#6]-[#8]-c1ccc(cc1-[#6]\[#6]=[#6](\[#6])-[#6])-[#6]-1-[#6]-[#6](=O)-c2ccc(-[#8]-[#6])cc2-[#8]-1 Show InChI InChI=1S/C23H26O5/c1-15(2)5-6-16-11-17(7-10-21(16)27-14-25-3)22-13-20(24)19-9-8-18(26-4)12-23(19)28-22/h5,7-12,22H,6,13-14H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human aromatase |
J Med Chem 50: 2799-806 (2007)
Article DOI: 10.1021/jm070109i
BindingDB Entry DOI: 10.7270/Q2668F0Z |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50213238

(7-methoxy-2-[4-(3-methylbut-2-enyloxy)phenyl]chrom...)Show SMILES [#6]-[#8]-c1ccc2-[#6](=O)-[#6]-[#6](-[#8]-c2c1)-c1ccc(-[#8]-[#6]\[#6]=[#6](\[#6])-[#6])cc1 Show InChI InChI=1S/C21H22O4/c1-14(2)10-11-24-16-6-4-15(5-7-16)20-13-19(22)18-9-8-17(23-3)12-21(18)25-20/h4-10,12,20H,11,13H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human aromatase |
J Med Chem 50: 2799-806 (2007)
Article DOI: 10.1021/jm070109i
BindingDB Entry DOI: 10.7270/Q2668F0Z |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50213251

((rac)-7-hydroxy-2-(4-hydroxy-3-(3-methylbut-2-enyl...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1cc(ccc1-[#8])-[#6]-1-[#6]-[#6](=O)-c2ccc(-[#8])cc2-[#8]-1 Show InChI InChI=1S/C20H20O4/c1-12(2)3-4-13-9-14(5-8-17(13)22)19-11-18(23)16-7-6-15(21)10-20(16)24-19/h3,5-10,19,21-22H,4,11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human aromatase |
J Med Chem 50: 2799-806 (2007)
Article DOI: 10.1021/jm070109i
BindingDB Entry DOI: 10.7270/Q2668F0Z |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50213248

(3-hydroxy-1-(2-hydroxy-4-methoxyphenyl)-3-[4-metho...)Show SMILES [#6]-[#8]-c1ccc(-[#6](=O)-[#6]-[#6](-[#8])-c2ccc(-[#8]-[#6])c(-[#6]\[#6]=[#6](\[#6])-[#6])c2)c(-[#8])c1 Show InChI InChI=1S/C22H26O5/c1-14(2)5-6-16-11-15(7-10-22(16)27-4)19(23)13-21(25)18-9-8-17(26-3)12-20(18)24/h5,7-12,19,23-24H,6,13H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 5.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human aromatase |
J Med Chem 50: 2799-806 (2007)
Article DOI: 10.1021/jm070109i
BindingDB Entry DOI: 10.7270/Q2668F0Z |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50213240
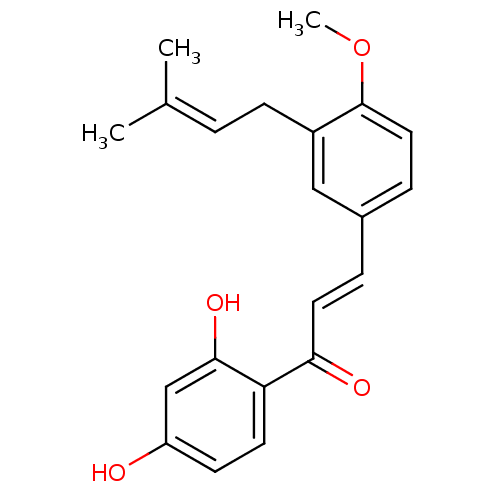
(1-(2,4-Dihydroxyphenyl)-3-[4-methoxy-3-(3-methylbu...)Show SMILES [#6]-[#8]-c1ccc(\[#6]=[#6]\[#6](=O)-c2ccc(-[#8])cc2-[#8])cc1-[#6]\[#6]=[#6](\[#6])-[#6] Show InChI InChI=1S/C21H22O4/c1-14(2)4-7-16-12-15(6-11-21(16)25-3)5-10-19(23)18-9-8-17(22)13-20(18)24/h4-6,8-13,22,24H,7H2,1-3H3/b10-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human aromatase |
J Med Chem 50: 2799-806 (2007)
Article DOI: 10.1021/jm070109i
BindingDB Entry DOI: 10.7270/Q2668F0Z |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50213244
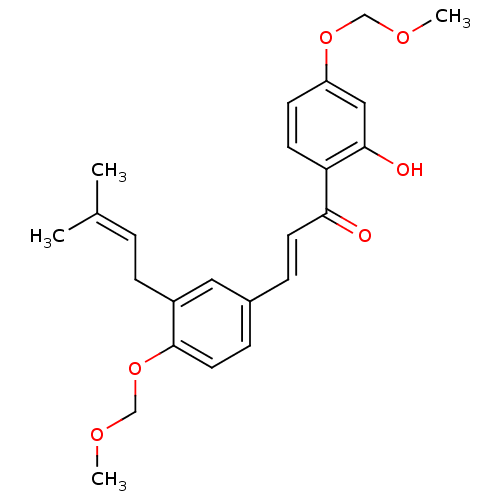
(1-[2-hydroxy-4-(methoxymethoxy)phenyl]-3-(4-[metho...)Show SMILES [#6]-[#8]-[#6]-[#8]-c1ccc(-[#6](=O)\[#6]=[#6]\c2ccc(-[#8]-[#6]-[#8]-[#6])c(-[#6]\[#6]=[#6](\[#6])-[#6])c2)c(-[#8])c1 Show InChI InChI=1S/C24H28O6/c1-17(2)5-8-19-13-18(7-12-24(19)30-16-28-4)6-11-22(25)21-10-9-20(14-23(21)26)29-15-27-3/h5-7,9-14,26H,8,15-16H2,1-4H3/b11-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.42E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human aromatase |
J Med Chem 50: 2799-806 (2007)
Article DOI: 10.1021/jm070109i
BindingDB Entry DOI: 10.7270/Q2668F0Z |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50213250
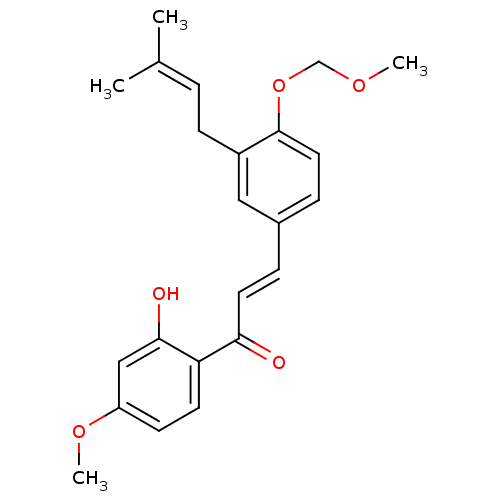
(1-(2-hydroxy-4-methoxyphenyl)-3-[4-(methoxymethoxy...)Show SMILES [#6]-[#8]-[#6]-[#8]-c1ccc(\[#6]=[#6]\[#6](=O)-c2ccc(-[#8]-[#6])cc2-[#8])cc1-[#6]\[#6]=[#6](\[#6])-[#6] Show InChI InChI=1S/C23H26O5/c1-16(2)5-8-18-13-17(7-12-23(18)28-15-26-3)6-11-21(24)20-10-9-19(27-4)14-22(20)25/h5-7,9-14,25H,8,15H2,1-4H3/b11-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.62E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human aromatase |
J Med Chem 50: 2799-806 (2007)
Article DOI: 10.1021/jm070109i
BindingDB Entry DOI: 10.7270/Q2668F0Z |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50213237
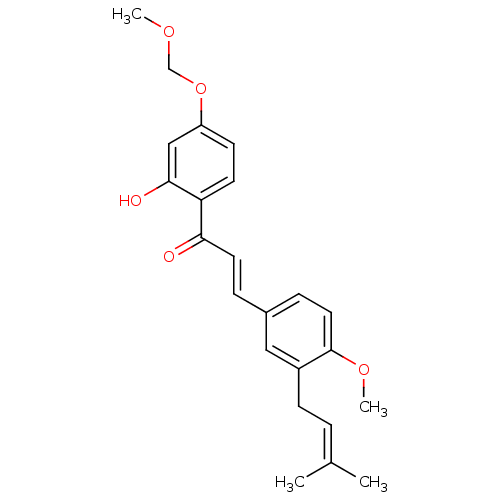
(1-[2-hydroxy-4-(methoxymethoxy)phenyl]-3-[4-methox...)Show SMILES [#6]-[#8]-[#6]-[#8]-c1ccc(-[#6](=O)\[#6]=[#6]\c2ccc(-[#8]-[#6])c(-[#6]\[#6]=[#6](\[#6])-[#6])c2)c(-[#8])c1 Show InChI InChI=1S/C23H26O5/c1-16(2)5-8-18-13-17(7-12-23(18)27-4)6-11-21(24)20-10-9-19(14-22(20)25)28-15-26-3/h5-7,9-14,25H,8,15H2,1-4H3/b11-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.62E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human aromatase |
J Med Chem 50: 2799-806 (2007)
Article DOI: 10.1021/jm070109i
BindingDB Entry DOI: 10.7270/Q2668F0Z |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50213249
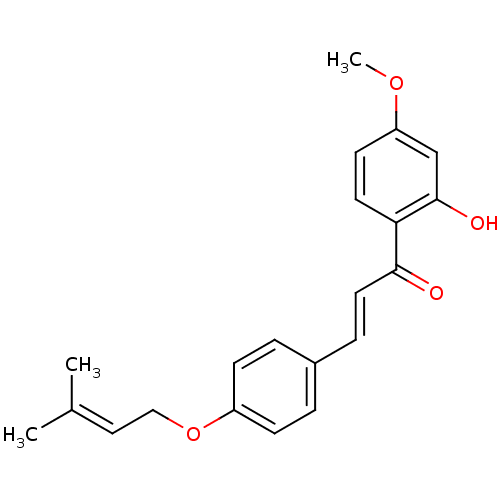
(1-(2-hydroxy-4-methoxyphenyl)-3-[4-(3-methylbut-2-...)Show SMILES [#6]-[#8]-c1ccc(-[#6](=O)\[#6]=[#6]\c2ccc(-[#8]-[#6]\[#6]=[#6](\[#6])-[#6])cc2)c(-[#8])c1 Show InChI InChI=1S/C21H22O4/c1-15(2)12-13-25-17-7-4-16(5-8-17)6-11-20(22)19-10-9-18(24-3)14-21(19)23/h4-12,14,23H,13H2,1-3H3/b11-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.71E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human aromatase |
J Med Chem 50: 2799-806 (2007)
Article DOI: 10.1021/jm070109i
BindingDB Entry DOI: 10.7270/Q2668F0Z |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50213233
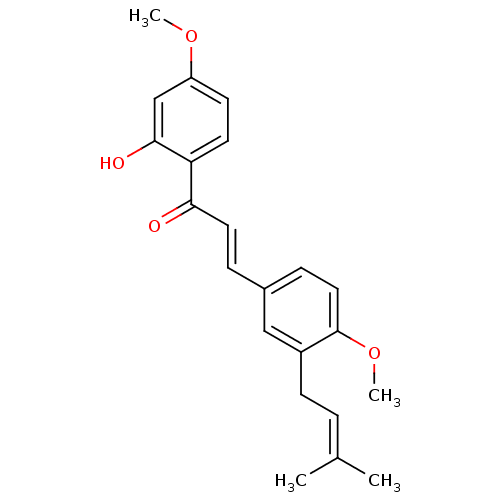
(1-(2-hydroxy-4-methoxyphenyl)-3-[4-methoxy-3-(3-me...)Show SMILES [#6]-[#8]-c1ccc(-[#6](=O)\[#6]=[#6]\c2ccc(-[#8]-[#6])c(-[#6]\[#6]=[#6](\[#6])-[#6])c2)c(-[#8])c1 Show InChI InChI=1S/C22H24O4/c1-15(2)5-8-17-13-16(7-12-22(17)26-4)6-11-20(23)19-10-9-18(25-3)14-21(19)24/h5-7,9-14,24H,8H2,1-4H3/b11-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.84E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human aromatase |
J Med Chem 50: 2799-806 (2007)
Article DOI: 10.1021/jm070109i
BindingDB Entry DOI: 10.7270/Q2668F0Z |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50213246
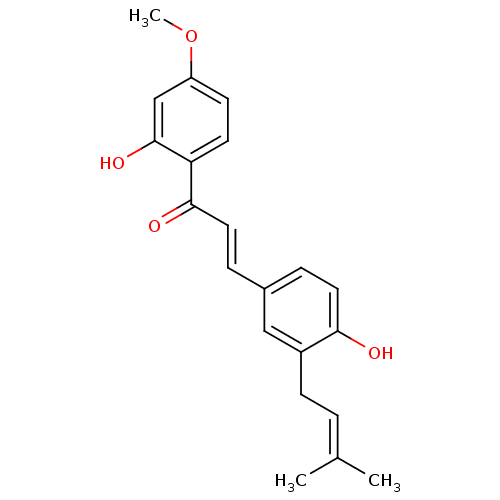
(3-[4-hydroxy-3-(3-methylbut-2-enyl)phenyl]-1-(2-hy...)Show SMILES [#6]-[#8]-c1ccc(-[#6](=O)\[#6]=[#6]\c2ccc(-[#8])c(-[#6]\[#6]=[#6](\[#6])-[#6])c2)c(-[#8])c1 Show InChI InChI=1S/C21H22O4/c1-14(2)4-7-16-12-15(5-10-19(16)22)6-11-20(23)18-9-8-17(25-3)13-21(18)24/h4-6,8-13,22,24H,7H2,1-3H3/b11-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.96E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human aromatase |
J Med Chem 50: 2799-806 (2007)
Article DOI: 10.1021/jm070109i
BindingDB Entry DOI: 10.7270/Q2668F0Z |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50212400
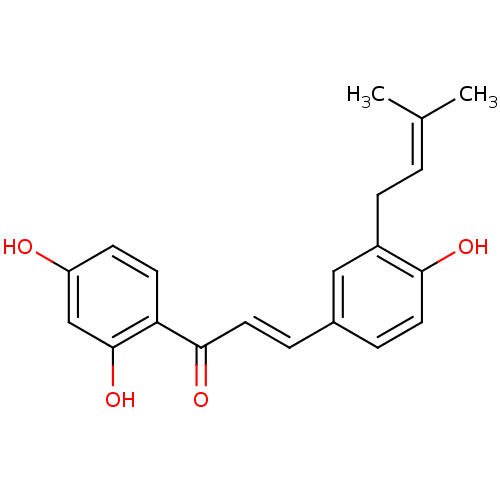
(1-(2,4-Dihydroxyphenyl)-3-[4-hydroxy-3-(3-methylbu...)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-c1cc(\[#6]=[#6]\[#6](=O)-c2ccc(-[#8])cc2-[#8])ccc1-[#8] Show InChI InChI=1S/C20H20O4/c1-13(2)3-6-15-11-14(4-9-18(15)22)5-10-19(23)17-8-7-16(21)12-20(17)24/h3-5,7-12,21-22,24H,6H2,1-2H3/b10-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.08E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human aromatase |
J Med Chem 50: 2799-806 (2007)
Article DOI: 10.1021/jm070109i
BindingDB Entry DOI: 10.7270/Q2668F0Z |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50213247
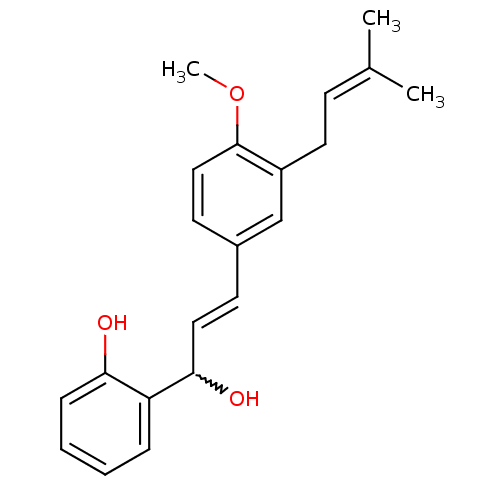
(1-(2-hydroxyphenyl)-3-[4-methoxy-3-(3-methylbut-2-...)Show SMILES [#6]-[#8]-c1ccc(\[#6]=[#6]\[#6](-[#8])-c2ccccc2-[#8])cc1-[#6]\[#6]=[#6](\[#6])-[#6] |w:8.8| Show InChI InChI=1S/C21H24O3/c1-15(2)8-11-17-14-16(10-13-21(17)24-3)9-12-20(23)18-6-4-5-7-19(18)22/h4-10,12-14,20,22-23H,11H2,1-3H3/b12-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human aromatase |
J Med Chem 50: 2799-806 (2007)
Article DOI: 10.1021/jm070109i
BindingDB Entry DOI: 10.7270/Q2668F0Z |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50213242
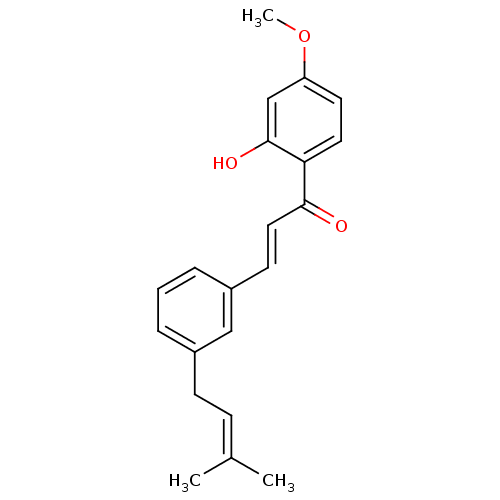
(1-(2-hydroxy-4-methoxyphenyl)-3-[3-(3-methylbut-2-...)Show SMILES [#6]-[#8]-c1ccc(-[#6](=O)\[#6]=[#6]\c2cccc(-[#6]\[#6]=[#6](\[#6])-[#6])c2)c(-[#8])c1 Show InChI InChI=1S/C21H22O3/c1-15(2)7-8-16-5-4-6-17(13-16)9-12-20(22)19-11-10-18(24-3)14-21(19)23/h4-7,9-14,23H,8H2,1-3H3/b12-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human aromatase |
J Med Chem 50: 2799-806 (2007)
Article DOI: 10.1021/jm070109i
BindingDB Entry DOI: 10.7270/Q2668F0Z |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50213232

(1-(2-hydroxy-4-methoxyphenyl)-3-(4-methoxyphenyl)p...)Show InChI InChI=1S/C17H16O4/c1-20-13-6-3-12(4-7-13)5-10-16(18)15-9-8-14(21-2)11-17(15)19/h3-11,19H,1-2H3/b10-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.52E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human aromatase |
J Med Chem 50: 2799-806 (2007)
Article DOI: 10.1021/jm070109i
BindingDB Entry DOI: 10.7270/Q2668F0Z |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data