

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50248410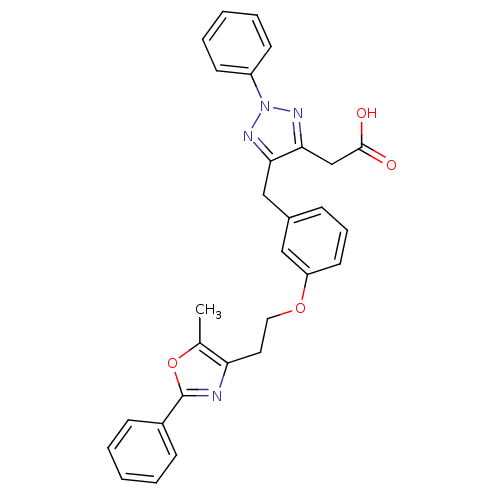 ((5-{3-[2-(5-methyl-2-phenyl-1,3-oxazol-4-yl)ethoxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to PPARgamma (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50248463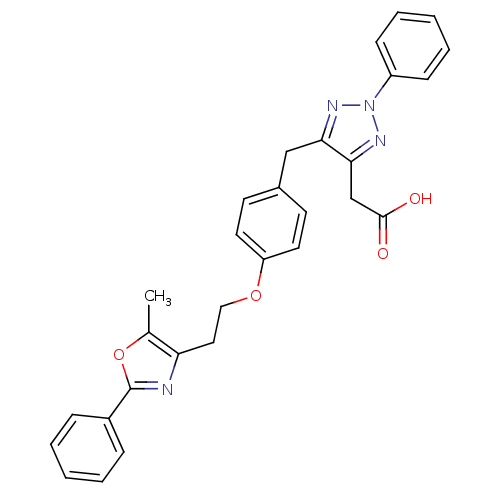 (2-(5-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)be...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to PPARgamma (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50248409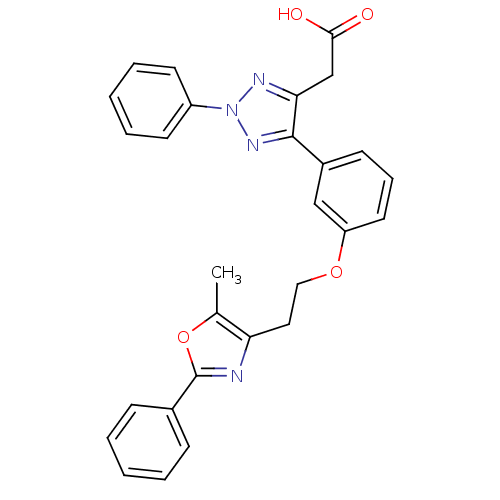 (2-(5-(3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to PPARgamma (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50248463 (2-(5-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)be...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to PPARalpha (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50248288 (5-(3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to PPARgamma (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50248288 (5-(3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to PPARalpha (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50248226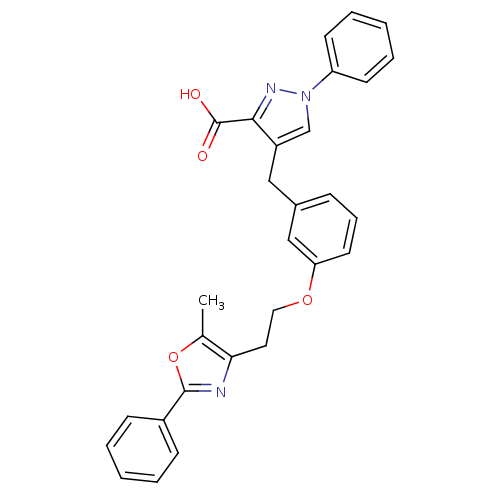 (4-(3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to PPARgamma (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50248224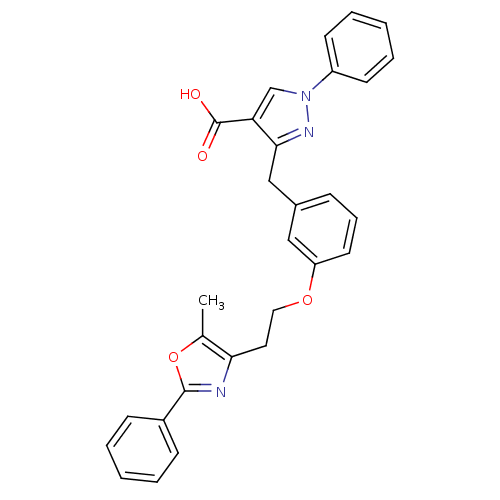 (3-(3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to PPARalpha (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50248410 ((5-{3-[2-(5-methyl-2-phenyl-1,3-oxazol-4-yl)ethoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to PPARalpha (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50248179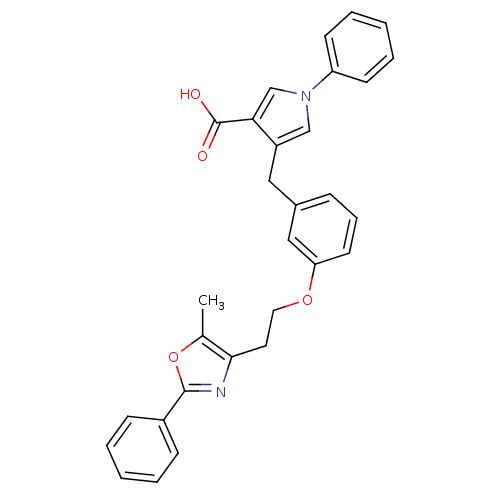 (4-(3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to PPARalpha (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50248224 (3-(3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to PPARgamma (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50248226 (4-(3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to PPARalpha (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50248179 (4-(3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to PPARgamma (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50248409 (2-(5-(3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to PPARalpha (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50248412 (2-(5-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 517 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to PPARgamma (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50248346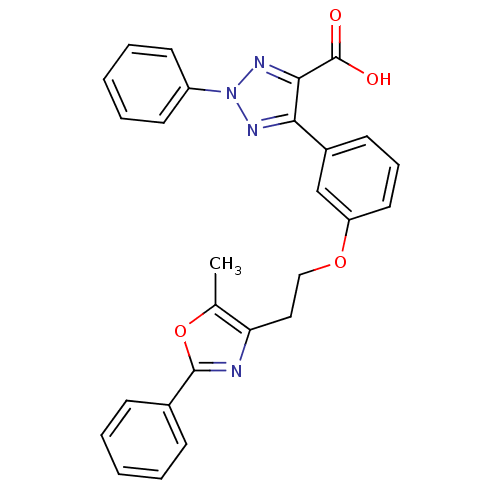 (5-(3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)pheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to PPARgamma (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50248343 (5-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 712 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to PPARgamma (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50248289 (5-(3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to PPARgamma (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50248345 (4-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to PPARgamma (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50248181 (4-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to PPARgamma (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50248181 (4-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to PPARalpha (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50248411 (5-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)pheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to PPARgamma (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50248290 (4-(3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to PPARgamma (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50248346 (5-(3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)pheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to PPARalpha (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50248178 (1-methyl-4-(3-(2-(5-methyl-2-phenyloxazol-4-yl)eth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to PPARgamma (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50248287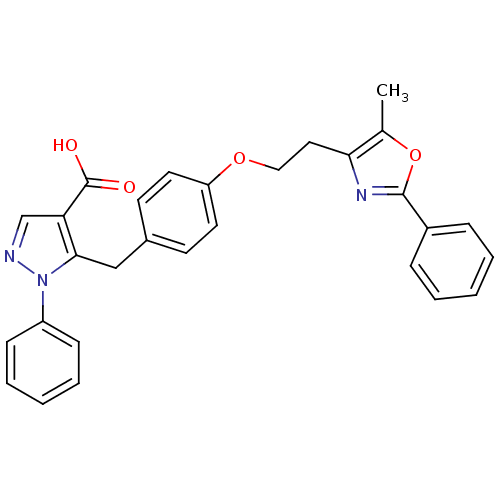 (5-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to PPARgamma (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50248227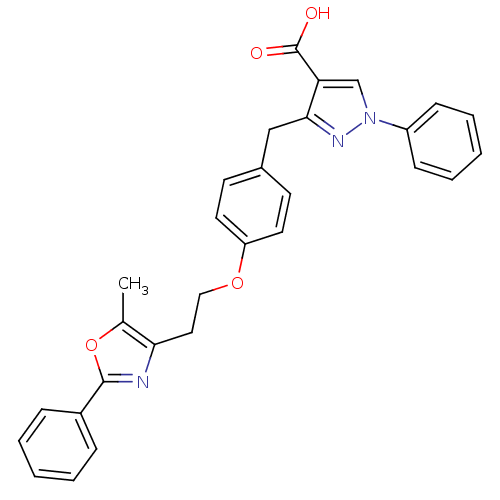 (3-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to PPARgamma (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50248227 (3-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to PPARalpha (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50248343 (5-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to PPARalpha (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50248412 (2-(5-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to PPARalpha (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50248180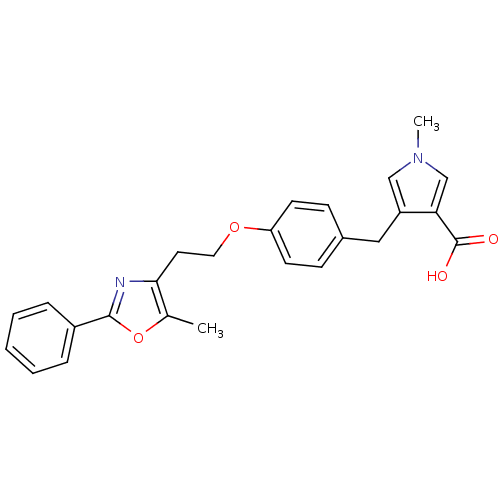 (1-methyl-4-(4-(2-(5-methyl-2-phenyloxazol-4-yl)eth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to PPARgamma (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50248225 (5-(3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to PPARgamma (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50248178 (1-methyl-4-(3-(2-(5-methyl-2-phenyloxazol-4-yl)eth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to PPARalpha (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50248290 (4-(3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to PPARalpha (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50248345 (4-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to PPARalpha (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50248344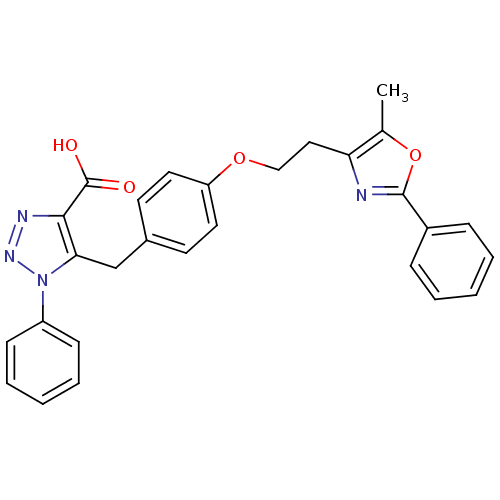 (5-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to PPARgamma (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50248180 (1-methyl-4-(4-(2-(5-methyl-2-phenyloxazol-4-yl)eth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to PPARalpha (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50248344 (5-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to PPARalpha (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50248289 (5-(3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to PPARalpha (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50248225 (5-(3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to PPARalpha (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50248287 (5-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to PPARalpha (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50248411 (5-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)pheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to PPARalpha (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50248288 (5-(3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARalpha ligand binding domain expressed in HEK293 cells by Gal4 transactivation assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50248343 (5-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.15E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARalpha ligand binding domain expressed in HEK293 cells by Gal4 transactivation assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50248344 (5-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARalpha ligand binding domain expressed in HEK293 cells by Gal4 transactivation assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50248179 (4-(3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 550 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARgamma ligand binding domain expressed in HEK293 cells by Gal4 transactivation assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50248181 (4-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARgamma ligand binding domain expressed in HEK293 cells by Gal4 transactivation assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50248287 (5-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARalpha ligand binding domain expressed in HEK293 cells by Gal4 transactivation assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50150998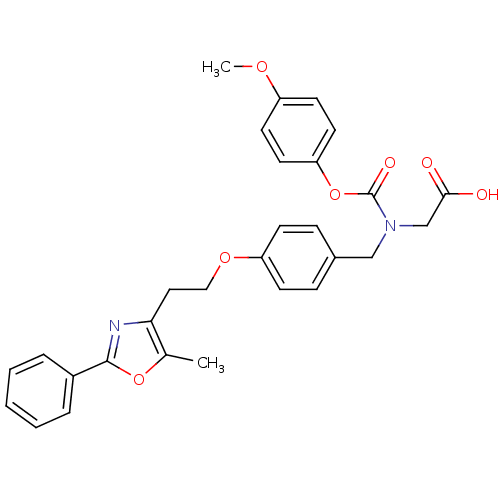 (((4-Methoxy-phenoxycarbonyl)-{4-[2-(5-methyl-2-phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to PPARalpha (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50248181 (4-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 660 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARalpha ligand binding domain expressed in HEK293 cells by Gal4 transactivation assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50248410 ((5-{3-[2-(5-methyl-2-phenyl-1,3-oxazol-4-yl)ethoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARalpha ligand binding domain expressed in HEK293 cells by Gal4 transactivation assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50248180 (1-methyl-4-(4-(2-(5-methyl-2-phenyloxazol-4-yl)eth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.57E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARgamma ligand binding domain expressed in HEK293 cells by Gal4 transactivation assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50248287 (5-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 490 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARgamma ligand binding domain expressed in HEK293 cells by Gal4 transactivation assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50248343 (5-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.27E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARgamma ligand binding domain expressed in HEK293 cells by Gal4 transactivation assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50248345 (4-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARgamma ligand binding domain expressed in HEK293 cells by Gal4 transactivation assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50248463 (2-(5-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)be...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARgamma ligand binding domain expressed in HEK293 cells by Gal4 transactivation assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50248180 (1-methyl-4-(4-(2-(5-methyl-2-phenyloxazol-4-yl)eth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARalpha ligand binding domain expressed in HEK293 cells by Gal4 transactivation assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50248224 (3-(3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARalpha ligand binding domain expressed in HEK293 cells by Gal4 transactivation assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50248225 (5-(3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 648 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARalpha ligand binding domain expressed in HEK293 cells by Gal4 transactivation assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50248226 (4-(3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 311 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARalpha ligand binding domain expressed in HEK293 cells by Gal4 transactivation assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50248227 (3-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 126 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARalpha ligand binding domain expressed in HEK293 cells by Gal4 transactivation assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50248288 (5-(3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.79E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARgamma ligand binding domain expressed in HEK293 cells by Gal4 transactivation assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50248346 (5-(3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)pheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.19E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARgamma ligand binding domain expressed in HEK293 cells by Gal4 transactivation assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50248178 (1-methyl-4-(3-(2-(5-methyl-2-phenyloxazol-4-yl)eth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.47E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARalpha ligand binding domain expressed in HEK293 cells by Gal4 transactivation assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50248463 (2-(5-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)be...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARalpha ligand binding domain expressed in HEK293 cells by Gal4 transactivation assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50248226 (4-(3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 156 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARgamma ligand binding domain expressed in HEK293 cells by Gal4 transactivation assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50248411 (5-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)pheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.33E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARgamma ligand binding domain expressed in HEK293 cells by Gal4 transactivation assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50150998 (((4-Methoxy-phenoxycarbonyl)-{4-[2-(5-methyl-2-phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 35 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to PPARgamma (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50248290 (4-(3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARalpha ligand binding domain expressed in HEK293 cells by Gal4 transactivation assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50248345 (4-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARalpha ligand binding domain expressed in HEK293 cells by Gal4 transactivation assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50248346 (5-(3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)pheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.02E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARalpha ligand binding domain expressed in HEK293 cells by Gal4 transactivation assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50248409 (2-(5-(3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 390 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARalpha ligand binding domain expressed in HEK293 cells by Gal4 transactivation assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50248178 (1-methyl-4-(3-(2-(5-methyl-2-phenyloxazol-4-yl)eth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.37E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARgamma ligand binding domain expressed in HEK293 cells by Gal4 transactivation assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50248344 (5-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.36E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARgamma ligand binding domain expressed in HEK293 cells by Gal4 transactivation assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50248410 ((5-{3-[2-(5-methyl-2-phenyl-1,3-oxazol-4-yl)ethoxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARgamma ligand binding domain expressed in HEK293 cells by Gal4 transactivation assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50248412 (2-(5-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.86E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARgamma ligand binding domain expressed in HEK293 cells by Gal4 transactivation assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50248179 (4-(3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 120 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARalpha ligand binding domain expressed in HEK293 cells by Gal4 transactivation assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50248289 (5-(3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARalpha ligand binding domain expressed in HEK293 cells by Gal4 transactivation assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50248412 (2-(5-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.14E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARalpha ligand binding domain expressed in HEK293 cells by Gal4 transactivation assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50248225 (5-(3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 437 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARgamma ligand binding domain expressed in HEK293 cells by Gal4 transactivation assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50248289 (5-(3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARgamma ligand binding domain expressed in HEK293 cells by Gal4 transactivation assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50248411 (5-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)pheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARalpha ligand binding domain expressed in HEK293 cells by Gal4 transactivation assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50248224 (3-(3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARgamma ligand binding domain expressed in HEK293 cells by Gal4 transactivation assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50248227 (3-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 752 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARgamma ligand binding domain expressed in HEK293 cells by Gal4 transactivation assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50248290 (4-(3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.27E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARgamma ligand binding domain expressed in HEK293 cells by Gal4 transactivation assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50248409 (2-(5-(3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 560 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARgamma ligand binding domain expressed in HEK293 cells by Gal4 transactivation assay | Bioorg Med Chem Lett 19: 1451-6 (2009) Article DOI: 10.1016/j.bmcl.2009.01.030 BindingDB Entry DOI: 10.7270/Q2MC8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||