

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50295811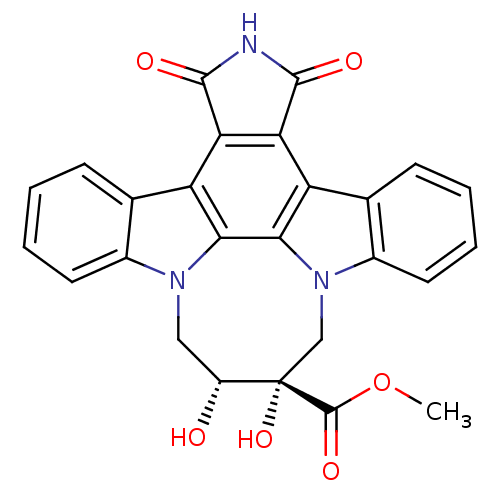 (12,13-[2-carboxymethyl-cis-2,3-dihydroxy)-1,4-buty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. 920 Route 202 Curated by ChEMBL | Assay Description Inhibition of JAK3 expressed in insect Sf21 cells assessed as inhibition of biotinylated substrate phosphorylation | Bioorg Med Chem Lett 19: 3333-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.039 BindingDB Entry DOI: 10.7270/Q2ZK5GQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50295819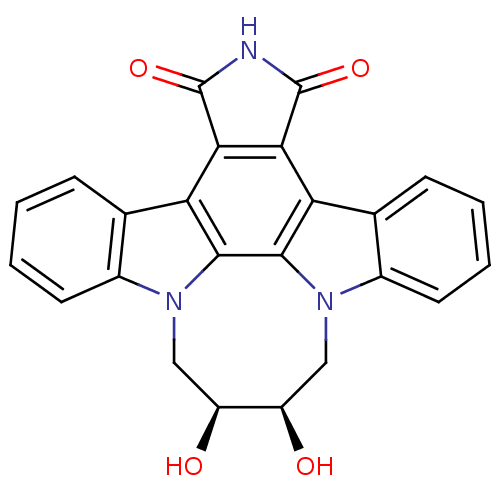 (12,13-(2,3-cis-dihydroxy-butan-1,4-yl)-12,13-dihyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. 920 Route 202 Curated by ChEMBL | Assay Description Inhibition of JAK3 expressed in insect Sf21 cells assessed as inhibition of biotinylated substrate phosphorylation | Bioorg Med Chem Lett 19: 3333-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.039 BindingDB Entry DOI: 10.7270/Q2ZK5GQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50295821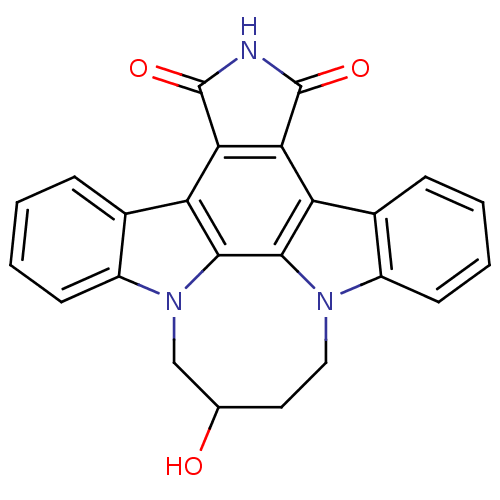 (12,13-(2-hydroxy-butan-1,4-yl)-12,13-dihydro-5,7-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. 920 Route 202 Curated by ChEMBL | Assay Description Inhibition of JAK3 expressed in insect Sf21 cells assessed as inhibition of biotinylated substrate phosphorylation | Bioorg Med Chem Lett 19: 3333-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.039 BindingDB Entry DOI: 10.7270/Q2ZK5GQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50295809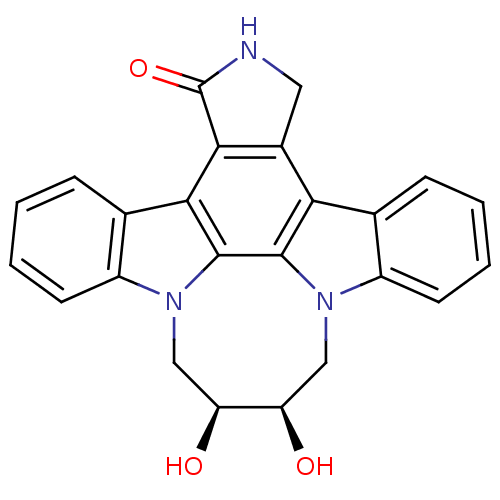 (12,13-(2,3-Cis-dihyroxy-1,4-butyl)-6,7,12,13-tetra...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. 920 Route 202 Curated by ChEMBL | Assay Description Inhibition of JAK3 expressed in insect Sf21 cells assessed as inhibition of biotinylated substrate phosphorylation | Bioorg Med Chem Lett 19: 3333-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.039 BindingDB Entry DOI: 10.7270/Q2ZK5GQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50059889 ((staurosporine)3-methoxy-2-methyl-4-methylamino-(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. 920 Route 202 Curated by ChEMBL | Assay Description Inhibition of JAK3 expressed in insect Sf21 cells assessed as inhibition of biotinylated substrate phosphorylation | Bioorg Med Chem Lett 19: 3333-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.039 BindingDB Entry DOI: 10.7270/Q2ZK5GQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50295810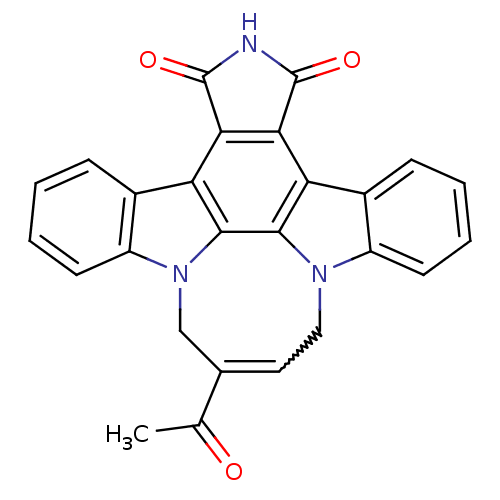 (12,13-[2-carbomethoxy-1,4-but-cis-2-enyl]-6,7,12,1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. 920 Route 202 Curated by ChEMBL | Assay Description Inhibition of JAK3 expressed in insect Sf21 cells assessed as inhibition of biotinylated substrate phosphorylation | Bioorg Med Chem Lett 19: 3333-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.039 BindingDB Entry DOI: 10.7270/Q2ZK5GQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50295820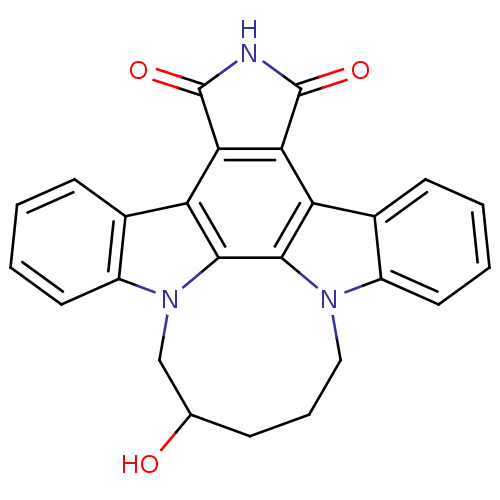 (12,13-(2-hydroxy-pentan-1,5-yl)-12,13-dihydro-5,7-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. 920 Route 202 Curated by ChEMBL | Assay Description Inhibition of JAK3 expressed in insect Sf21 cells assessed as inhibition of biotinylated substrate phosphorylation | Bioorg Med Chem Lett 19: 3333-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.039 BindingDB Entry DOI: 10.7270/Q2ZK5GQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50295817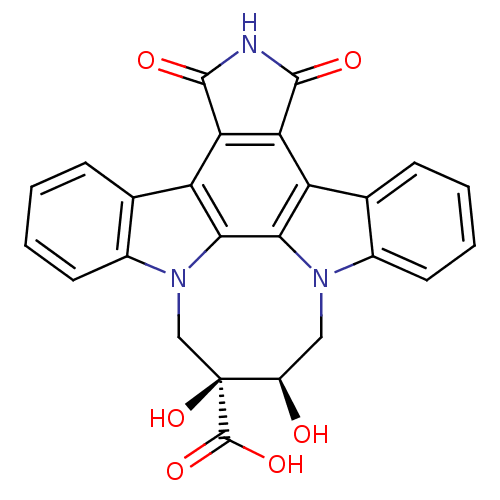 (12,13-[2-carboxy-cis-2,3-dihydroxy-1,4-butyl]-6,7,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. 920 Route 202 Curated by ChEMBL | Assay Description Inhibition of JAK3 expressed in insect Sf21 cells assessed as inhibition of biotinylated substrate phosphorylation | Bioorg Med Chem Lett 19: 3333-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.039 BindingDB Entry DOI: 10.7270/Q2ZK5GQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50295818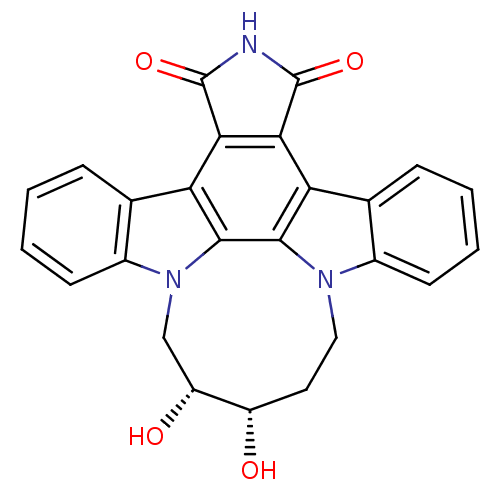 (12,13-(2,3-cis-dihydroxy-pentan-1,5-yl)-12,13-dihy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. 920 Route 202 Curated by ChEMBL | Assay Description Inhibition of JAK3 expressed in insect Sf21 cells assessed as inhibition of biotinylated substrate phosphorylation | Bioorg Med Chem Lett 19: 3333-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.039 BindingDB Entry DOI: 10.7270/Q2ZK5GQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50295812 (12,13-(3,4-Cis-dihyroxy-1,6-hexyl)-6,7,12,13-tetra...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. 920 Route 202 Curated by ChEMBL | Assay Description Inhibition of JAK3 expressed in insect Sf21 cells assessed as inhibition of biotinylated substrate phosphorylation | Bioorg Med Chem Lett 19: 3333-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.039 BindingDB Entry DOI: 10.7270/Q2ZK5GQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50295816 ((16S,17R)-16-{[3-(dimethylamino)pyrrolidin-1-yl]ca...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. 920 Route 202 Curated by ChEMBL | Assay Description Inhibition of JAK3 expressed in insect Sf21 cells assessed as inhibition of biotinylated substrate phosphorylation | Bioorg Med Chem Lett 19: 3333-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.039 BindingDB Entry DOI: 10.7270/Q2ZK5GQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50295813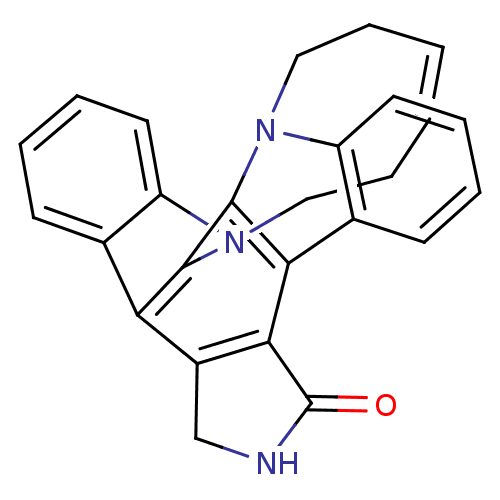 (12,13-(1,6-hex-cis-3-enyl)-6,7,12,13-tetrahydro-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. 920 Route 202 Curated by ChEMBL | Assay Description Inhibition of JAK3 expressed in insect Sf21 cells assessed as inhibition of biotinylated substrate phosphorylation | Bioorg Med Chem Lett 19: 3333-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.039 BindingDB Entry DOI: 10.7270/Q2ZK5GQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50295814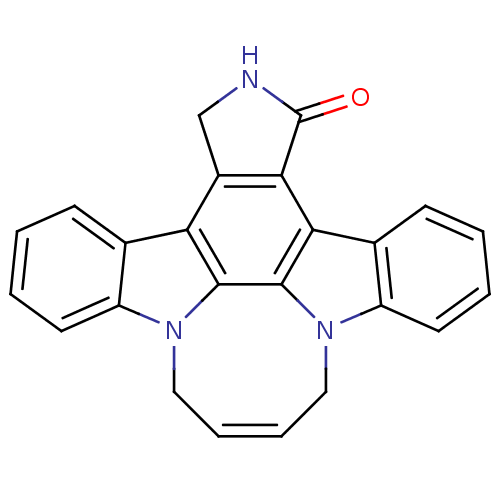 (12,13-(1,4-but-cis-2-enyl)-6,7,12,13-tetrahydro-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. 920 Route 202 Curated by ChEMBL | Assay Description Inhibition of JAK3 expressed in insect Sf21 cells assessed as inhibition of biotinylated substrate phosphorylation | Bioorg Med Chem Lett 19: 3333-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.039 BindingDB Entry DOI: 10.7270/Q2ZK5GQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM2581 (3,13,23-triazahexacyclo[14.7.0.0^{2,10}.0^{4,9}.0^...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. 920 Route 202 Curated by ChEMBL | Assay Description Inhibition of JAK3 expressed in insect Sf21 cells assessed as inhibition of biotinylated substrate phosphorylation | Bioorg Med Chem Lett 19: 3333-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.039 BindingDB Entry DOI: 10.7270/Q2ZK5GQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ZAP-70 (Homo sapiens (Human)) | BDBM50295809 (12,13-(2,3-Cis-dihyroxy-1,4-butyl)-6,7,12,13-tetra...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. 920 Route 202 Curated by ChEMBL | Assay Description Inhibition of Zap70-mediated inhibition of biotinylated substrate phosphorylation | Bioorg Med Chem Lett 19: 3333-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.039 BindingDB Entry DOI: 10.7270/Q2ZK5GQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50295815 (12,13-diallyl-6,7,12,13-tetrahydro-5-oxo-5H-indolo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >200 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. 920 Route 202 Curated by ChEMBL | Assay Description Inhibition of JAK3 expressed in insect Sf21 cells assessed as inhibition of biotinylated substrate phosphorylation | Bioorg Med Chem Lett 19: 3333-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.039 BindingDB Entry DOI: 10.7270/Q2ZK5GQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50295811 (12,13-[2-carboxymethyl-cis-2,3-dihydroxy)-1,4-buty...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. 920 Route 202 Curated by ChEMBL | Assay Description Inhibition of LCK-mediated inhibition of biotinylated substrate phosphorylation | Bioorg Med Chem Lett 19: 3333-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.039 BindingDB Entry DOI: 10.7270/Q2ZK5GQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50295809 (12,13-(2,3-Cis-dihyroxy-1,4-butyl)-6,7,12,13-tetra...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. 920 Route 202 Curated by ChEMBL | Assay Description Inhibition of SYK-mediated inhibition of biotinylated substrate phosphorylation | Bioorg Med Chem Lett 19: 3333-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.039 BindingDB Entry DOI: 10.7270/Q2ZK5GQ8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| MAP kinase-activated protein kinase 2 (Homo sapiens (Human)) | BDBM50295810 (12,13-[2-carbomethoxy-1,4-but-cis-2-enyl]-6,7,12,1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. 920 Route 202 Curated by ChEMBL | Assay Description Inhibition of MK2-mediated inhibition of biotinylated substrate phosphorylation | Bioorg Med Chem Lett 19: 3333-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.039 BindingDB Entry DOI: 10.7270/Q2ZK5GQ8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Signal transducer and activator of transcription 5A (Homo sapiens (Human)) | BDBM50295809 (12,13-(2,3-Cis-dihyroxy-1,4-butyl)-6,7,12,13-tetra...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 689 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. 920 Route 202 Curated by ChEMBL | Assay Description Inhibition of STAT5 phosphorylation in IL2-stimulated healthy human T cells by western blotting | Bioorg Med Chem Lett 19: 3333-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.039 BindingDB Entry DOI: 10.7270/Q2ZK5GQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50295811 (12,13-[2-carboxymethyl-cis-2,3-dihydroxy)-1,4-buty...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. 920 Route 202 Curated by ChEMBL | Assay Description Inhibition of SYK-mediated inhibition of biotinylated substrate phosphorylation | Bioorg Med Chem Lett 19: 3333-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.039 BindingDB Entry DOI: 10.7270/Q2ZK5GQ8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50295809 (12,13-(2,3-Cis-dihyroxy-1,4-butyl)-6,7,12,13-tetra...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. 920 Route 202 Curated by ChEMBL | Assay Description Inhibition of LCK-mediated inhibition of biotinylated substrate phosphorylation | Bioorg Med Chem Lett 19: 3333-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.039 BindingDB Entry DOI: 10.7270/Q2ZK5GQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50295810 (12,13-[2-carbomethoxy-1,4-but-cis-2-enyl]-6,7,12,1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. 920 Route 202 Curated by ChEMBL | Assay Description Inhibition of LCK-mediated inhibition of biotinylated substrate phosphorylation | Bioorg Med Chem Lett 19: 3333-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.039 BindingDB Entry DOI: 10.7270/Q2ZK5GQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50295810 (12,13-[2-carbomethoxy-1,4-but-cis-2-enyl]-6,7,12,1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. 920 Route 202 Curated by ChEMBL | Assay Description Inhibition of SYK-mediated inhibition of biotinylated substrate phosphorylation | Bioorg Med Chem Lett 19: 3333-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.039 BindingDB Entry DOI: 10.7270/Q2ZK5GQ8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein kinase ZAP-70 (Homo sapiens (Human)) | BDBM50295810 (12,13-[2-carbomethoxy-1,4-but-cis-2-enyl]-6,7,12,1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. 920 Route 202 Curated by ChEMBL | Assay Description Inhibition of Zap70-mediated inhibition of biotinylated substrate phosphorylation | Bioorg Med Chem Lett 19: 3333-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.039 BindingDB Entry DOI: 10.7270/Q2ZK5GQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ZAP-70 (Homo sapiens (Human)) | BDBM50295811 (12,13-[2-carboxymethyl-cis-2,3-dihydroxy)-1,4-buty...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. 920 Route 202 Curated by ChEMBL | Assay Description Inhibition of Zap70-mediated inhibition of biotinylated substrate phosphorylation | Bioorg Med Chem Lett 19: 3333-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.039 BindingDB Entry DOI: 10.7270/Q2ZK5GQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-activated protein kinase 2 (Homo sapiens (Human)) | BDBM50295811 (12,13-[2-carboxymethyl-cis-2,3-dihydroxy)-1,4-buty...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. 920 Route 202 Curated by ChEMBL | Assay Description Inhibition of MK2-mediated inhibition of biotinylated substrate phosphorylation | Bioorg Med Chem Lett 19: 3333-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.039 BindingDB Entry DOI: 10.7270/Q2ZK5GQ8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| MAP kinase-activated protein kinase 2 (Homo sapiens (Human)) | BDBM50295809 (12,13-(2,3-Cis-dihyroxy-1,4-butyl)-6,7,12,13-tetra...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. 920 Route 202 Curated by ChEMBL | Assay Description Inhibition of MK2-mediated inhibition of biotinylated substrate phosphorylation | Bioorg Med Chem Lett 19: 3333-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.039 BindingDB Entry DOI: 10.7270/Q2ZK5GQ8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||