

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50300676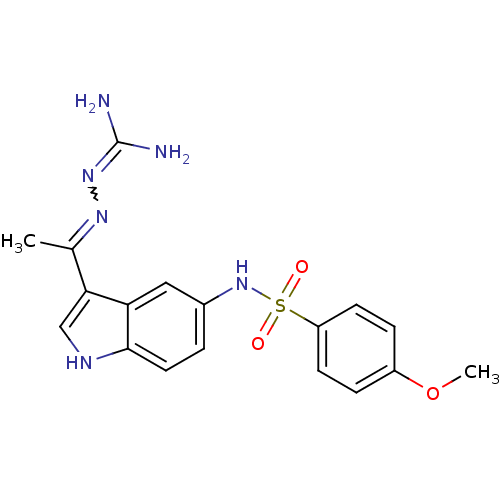 (2-(1-(5-(4-methoxyphenylsulfonamido)-1H-indol-3-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human cloned 5HT6 receptor expressed in human HeLa cells | J Med Chem 52: 6153-7 (2009) Article DOI: 10.1021/jm900796p BindingDB Entry DOI: 10.7270/Q2BG2P3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50300675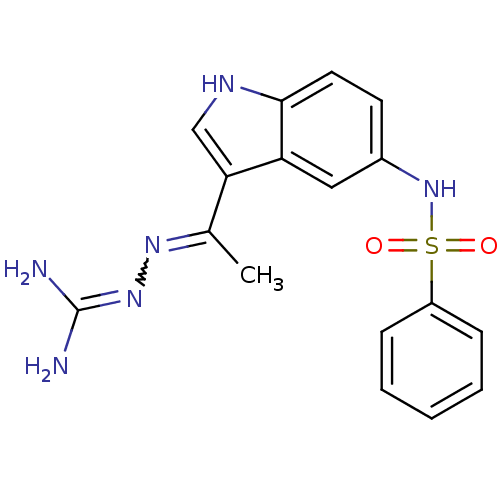 (2-(1-(5-(phenylsulfonamido)-1H-indol-3-yl)ethylide...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human cloned 5HT6 receptor expressed in human HeLa cells | J Med Chem 52: 6153-7 (2009) Article DOI: 10.1021/jm900796p BindingDB Entry DOI: 10.7270/Q2BG2P3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50300669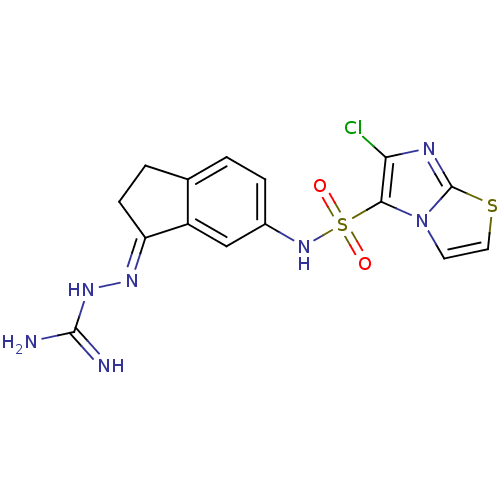 (2-(6-{[(6-Chloroimidazo[2,1-b][1,3]thiazol-5-yl)su...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells after 60 mins by liquid scintillation counting | J Med Chem 52: 6153-7 (2009) Article DOI: 10.1021/jm900796p BindingDB Entry DOI: 10.7270/Q2BG2P3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50300670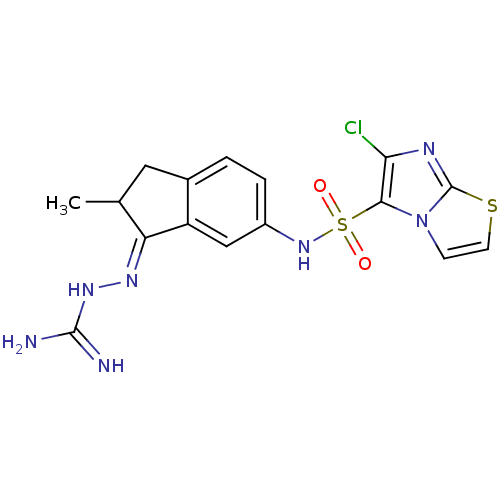 ((+/-)-2-(6-{[(6-Chloroimidazo[2,1-b][1,3]thiazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells after 60 mins by liquid scintillation counting | J Med Chem 52: 6153-7 (2009) Article DOI: 10.1021/jm900796p BindingDB Entry DOI: 10.7270/Q2BG2P3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21358 (2-[1-({6-chloroimidazo[2,1-b][1,3]thiazole-5-}sulf...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human cloned 5HT6 receptor expressed in human HeLa cells | J Med Chem 52: 6153-7 (2009) Article DOI: 10.1021/jm900796p BindingDB Entry DOI: 10.7270/Q2BG2P3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM34152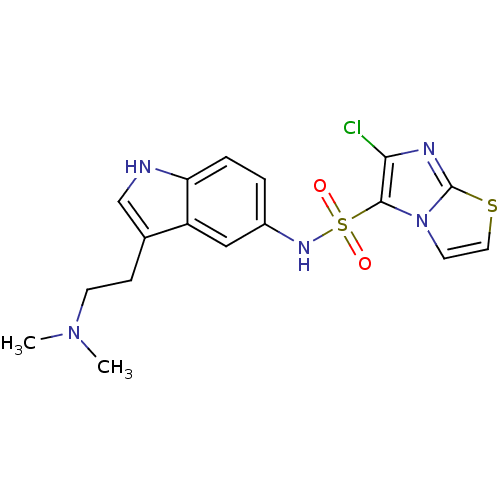 (CHEMBL362628 | E-6801) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Binding affinity to 5HT6 receptor | J Med Chem 52: 6153-7 (2009) Article DOI: 10.1021/jm900796p BindingDB Entry DOI: 10.7270/Q2BG2P3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50300674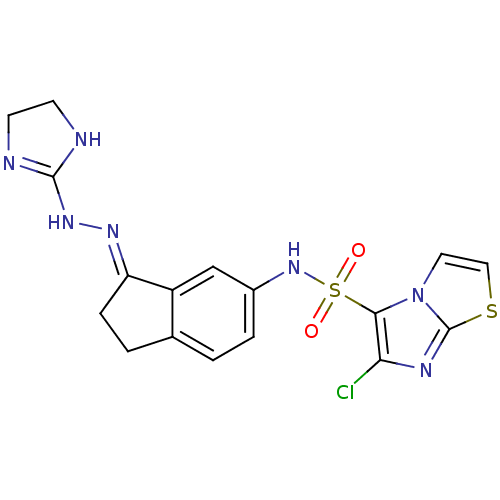 (6-chloro-N-[3-(4,5-dihydro-1H-imidazol-2-ylhydrazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells after 60 mins by liquid scintillation counting | J Med Chem 52: 6153-7 (2009) Article DOI: 10.1021/jm900796p BindingDB Entry DOI: 10.7270/Q2BG2P3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50300671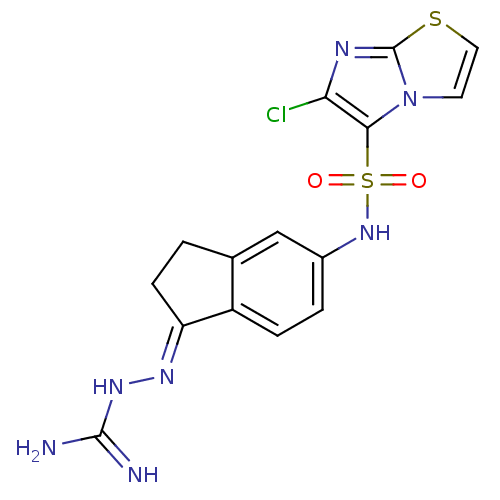 (2-(5-{[(6-Chloroimidazo[2,1-b][1,3]thiazol-5-yl)su...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells after 60 mins by liquid scintillation counting | J Med Chem 52: 6153-7 (2009) Article DOI: 10.1021/jm900796p BindingDB Entry DOI: 10.7270/Q2BG2P3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM34156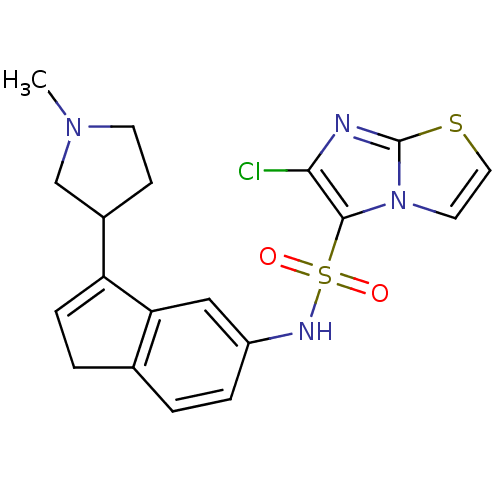 (indenylsulfonamide, 28) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells after 60 mins by liquid scintillation counting | J Med Chem 52: 6153-7 (2009) Article DOI: 10.1021/jm900796p BindingDB Entry DOI: 10.7270/Q2BG2P3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50300673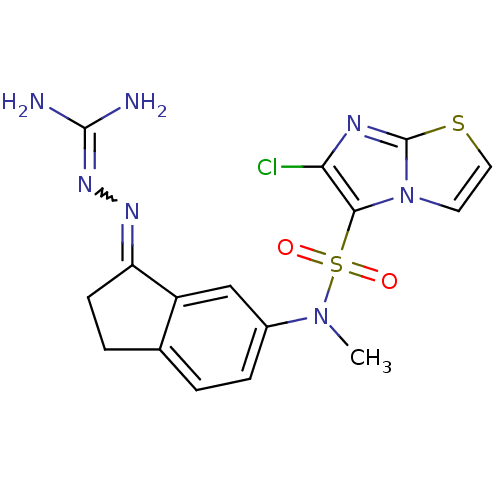 (2-(6-{[(6-chloroimidazo[2,1-b][1,3]thiazol-5-yl)su...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells after 60 mins by liquid scintillation counting | J Med Chem 52: 6153-7 (2009) Article DOI: 10.1021/jm900796p BindingDB Entry DOI: 10.7270/Q2BG2P3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50300672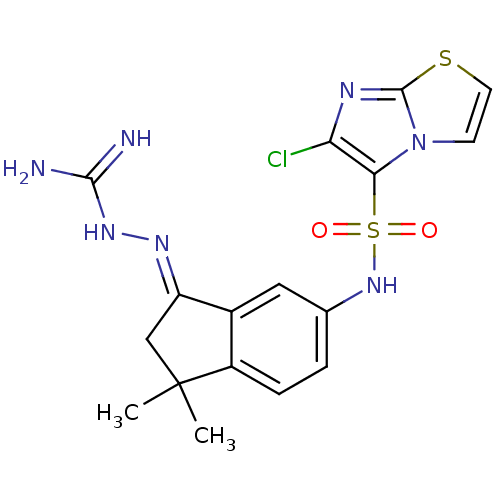 (2-(6-{[(6-chloroimidazo[2,1-b][1,3]thiazol-5-yl)su...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells after 60 mins by liquid scintillation counting | J Med Chem 52: 6153-7 (2009) Article DOI: 10.1021/jm900796p BindingDB Entry DOI: 10.7270/Q2BG2P3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50300667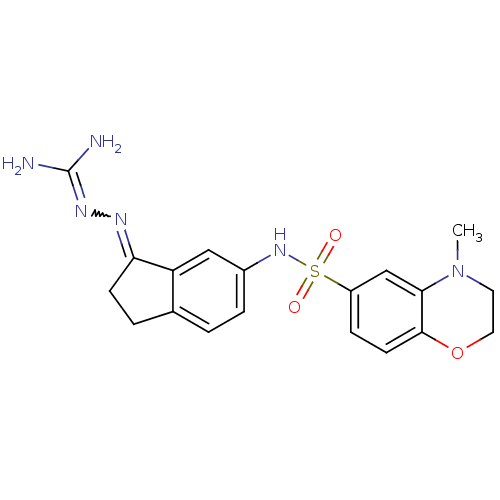 (2-(6-{[(4-methyl-3,4-dihydro-2H-1,4-benzoxazine-7-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells after 60 mins by liquid scintillation counting | J Med Chem 52: 6153-7 (2009) Article DOI: 10.1021/jm900796p BindingDB Entry DOI: 10.7270/Q2BG2P3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50300666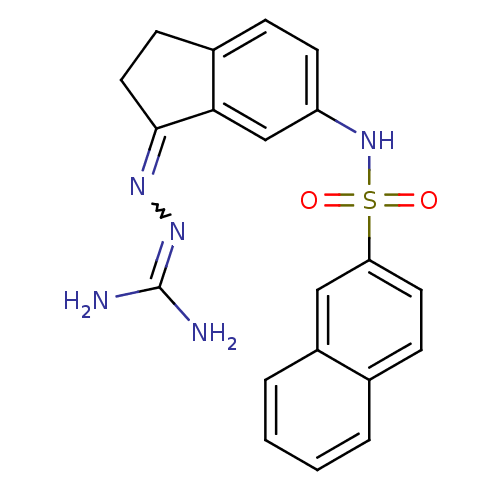 (2-{6-[(2-Naphthylsulfonyl)amino]-2,3-dihydro-1H-in...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells after 60 mins by liquid scintillation counting | J Med Chem 52: 6153-7 (2009) Article DOI: 10.1021/jm900796p BindingDB Entry DOI: 10.7270/Q2BG2P3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50300668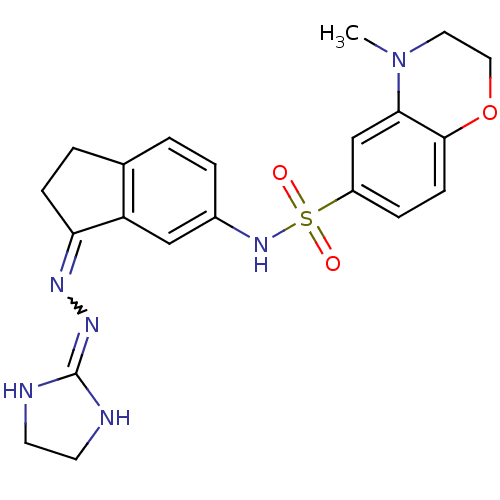 (CHEMBL576967 | N-[3-(4,5-dihydro-1H-imidazol-2-ylh...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells after 60 mins by liquid scintillation counting | J Med Chem 52: 6153-7 (2009) Article DOI: 10.1021/jm900796p BindingDB Entry DOI: 10.7270/Q2BG2P3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50300671 (2-(5-{[(6-Chloroimidazo[2,1-b][1,3]thiazol-5-yl)su...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Antagonist activity at human 5HT6 receptor expressed in HEK293F cells assessed as inhibition of 5HT-stimulated cAMP production after 30 mins by HTRF ... | J Med Chem 52: 6153-7 (2009) Article DOI: 10.1021/jm900796p BindingDB Entry DOI: 10.7270/Q2BG2P3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50300666 (2-{6-[(2-Naphthylsulfonyl)amino]-2,3-dihydro-1H-in...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Antagonist activity at human 5HT6 receptor expressed in HEK293F cells assessed as inhibition of 5HT-stimulated cAMP production after 30 mins by HTRF ... | J Med Chem 52: 6153-7 (2009) Article DOI: 10.1021/jm900796p BindingDB Entry DOI: 10.7270/Q2BG2P3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50300673 (2-(6-{[(6-chloroimidazo[2,1-b][1,3]thiazol-5-yl)su...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Antagonist activity at human 5HT6 receptor expressed in HEK293F cells assessed as inhibition of 5HT-stimulated cAMP production after 30 mins by HTRF ... | J Med Chem 52: 6153-7 (2009) Article DOI: 10.1021/jm900796p BindingDB Entry DOI: 10.7270/Q2BG2P3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50300670 ((+/-)-2-(6-{[(6-Chloroimidazo[2,1-b][1,3]thiazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Antagonist activity at human 5HT6 receptor expressed in HEK293F cells assessed as inhibition of 5HT-stimulated cAMP production after 30 mins by HTRF ... | J Med Chem 52: 6153-7 (2009) Article DOI: 10.1021/jm900796p BindingDB Entry DOI: 10.7270/Q2BG2P3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50300669 (2-(6-{[(6-Chloroimidazo[2,1-b][1,3]thiazol-5-yl)su...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Antagonist activity at human 5HT6 receptor expressed in HEK293F cells assessed as inhibition of 5HT-stimulated cAMP production after 30 mins by HTRF ... | J Med Chem 52: 6153-7 (2009) Article DOI: 10.1021/jm900796p BindingDB Entry DOI: 10.7270/Q2BG2P3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50300674 (6-chloro-N-[3-(4,5-dihydro-1H-imidazol-2-ylhydrazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 176 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Antagonist activity at human 5HT6 receptor expressed in HEK293F cells assessed as inhibition of 5HT-stimulated cAMP production after 30 mins by HTRF ... | J Med Chem 52: 6153-7 (2009) Article DOI: 10.1021/jm900796p BindingDB Entry DOI: 10.7270/Q2BG2P3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50300672 (2-(6-{[(6-chloroimidazo[2,1-b][1,3]thiazol-5-yl)su...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 238 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Antagonist activity at human 5HT6 receptor expressed in HEK293F cells assessed as inhibition of 5HT-stimulated cAMP production after 30 mins by HTRF ... | J Med Chem 52: 6153-7 (2009) Article DOI: 10.1021/jm900796p BindingDB Entry DOI: 10.7270/Q2BG2P3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50300667 (2-(6-{[(4-methyl-3,4-dihydro-2H-1,4-benzoxazine-7-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 581 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Antagonist activity at human 5HT6 receptor expressed in HEK293F cells assessed as inhibition of 5HT-stimulated cAMP production after 30 mins by HTRF ... | J Med Chem 52: 6153-7 (2009) Article DOI: 10.1021/jm900796p BindingDB Entry DOI: 10.7270/Q2BG2P3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50300670 ((+/-)-2-(6-{[(6-Chloroimidazo[2,1-b][1,3]thiazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human 5HT2C receptor | J Med Chem 52: 6153-7 (2009) Article DOI: 10.1021/jm900796p BindingDB Entry DOI: 10.7270/Q2BG2P3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50300672 (2-(6-{[(6-chloroimidazo[2,1-b][1,3]thiazol-5-yl)su...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human 5HT2C receptor | J Med Chem 52: 6153-7 (2009) Article DOI: 10.1021/jm900796p BindingDB Entry DOI: 10.7270/Q2BG2P3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50300667 (2-(6-{[(4-methyl-3,4-dihydro-2H-1,4-benzoxazine-7-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human 5HT1A receptor | J Med Chem 52: 6153-7 (2009) Article DOI: 10.1021/jm900796p BindingDB Entry DOI: 10.7270/Q2BG2P3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50300673 (2-(6-{[(6-chloroimidazo[2,1-b][1,3]thiazol-5-yl)su...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human 5HT1A receptor | J Med Chem 52: 6153-7 (2009) Article DOI: 10.1021/jm900796p BindingDB Entry DOI: 10.7270/Q2BG2P3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50300670 ((+/-)-2-(6-{[(6-Chloroimidazo[2,1-b][1,3]thiazol-5...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human SERT | J Med Chem 52: 6153-7 (2009) Article DOI: 10.1021/jm900796p BindingDB Entry DOI: 10.7270/Q2BG2P3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50300666 (2-{6-[(2-Naphthylsulfonyl)amino]-2,3-dihydro-1H-in...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human 5HT1A receptor | J Med Chem 52: 6153-7 (2009) Article DOI: 10.1021/jm900796p BindingDB Entry DOI: 10.7270/Q2BG2P3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50300670 ((+/-)-2-(6-{[(6-Chloroimidazo[2,1-b][1,3]thiazol-5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human 5HT1A receptor | J Med Chem 52: 6153-7 (2009) Article DOI: 10.1021/jm900796p BindingDB Entry DOI: 10.7270/Q2BG2P3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50300672 (2-(6-{[(6-chloroimidazo[2,1-b][1,3]thiazol-5-yl)su...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human 5HT1A receptor | J Med Chem 52: 6153-7 (2009) Article DOI: 10.1021/jm900796p BindingDB Entry DOI: 10.7270/Q2BG2P3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM34156 (indenylsulfonamide, 28) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Antagonist activity at human 5HT6 receptor expressed in HEK293F cells assessed as inhibition of 5HT-stimulated cAMP production after 30 mins by HTRF ... | J Med Chem 52: 6153-7 (2009) Article DOI: 10.1021/jm900796p BindingDB Entry DOI: 10.7270/Q2BG2P3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50300672 (2-(6-{[(6-chloroimidazo[2,1-b][1,3]thiazol-5-yl)su...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human SERT | J Med Chem 52: 6153-7 (2009) Article DOI: 10.1021/jm900796p BindingDB Entry DOI: 10.7270/Q2BG2P3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50300671 (2-(5-{[(6-Chloroimidazo[2,1-b][1,3]thiazol-5-yl)su...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human 5HT1A receptor | J Med Chem 52: 6153-7 (2009) Article DOI: 10.1021/jm900796p BindingDB Entry DOI: 10.7270/Q2BG2P3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50300669 (2-(6-{[(6-Chloroimidazo[2,1-b][1,3]thiazol-5-yl)su...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human 5HT1A receptor | J Med Chem 52: 6153-7 (2009) Article DOI: 10.1021/jm900796p BindingDB Entry DOI: 10.7270/Q2BG2P3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50300674 (6-chloro-N-[3-(4,5-dihydro-1H-imidazol-2-ylhydrazo...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human SERT | J Med Chem 52: 6153-7 (2009) Article DOI: 10.1021/jm900796p BindingDB Entry DOI: 10.7270/Q2BG2P3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50300669 (2-(6-{[(6-Chloroimidazo[2,1-b][1,3]thiazol-5-yl)su...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human 5HT2C receptor | J Med Chem 52: 6153-7 (2009) Article DOI: 10.1021/jm900796p BindingDB Entry DOI: 10.7270/Q2BG2P3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50300669 (2-(6-{[(6-Chloroimidazo[2,1-b][1,3]thiazol-5-yl)su...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human SERT | J Med Chem 52: 6153-7 (2009) Article DOI: 10.1021/jm900796p BindingDB Entry DOI: 10.7270/Q2BG2P3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50300674 (6-chloro-N-[3-(4,5-dihydro-1H-imidazol-2-ylhydrazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human 5HT2C receptor | J Med Chem 52: 6153-7 (2009) Article DOI: 10.1021/jm900796p BindingDB Entry DOI: 10.7270/Q2BG2P3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21358 (2-[1-({6-chloroimidazo[2,1-b][1,3]thiazole-5-}sulf...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Agonist activity at human 5HT6 receptor expressed in HeLa cells assessed as induction of cAMP production after 10 mins by radioimmunoassay | J Med Chem 52: 6153-7 (2009) Article DOI: 10.1021/jm900796p BindingDB Entry DOI: 10.7270/Q2BG2P3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||