

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50314762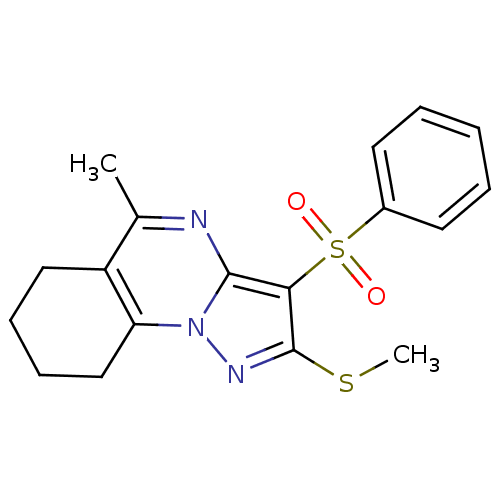 (5-methyl-2-(methylthio)-3-(phenylsulfonyl)-6,7,8,9...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]LSD from 5HT6 receptor in humanHeLa cells after 120 mins | Bioorg Med Chem Lett 20: 2133-6 (2010) Article DOI: 10.1016/j.bmcl.2010.02.046 BindingDB Entry DOI: 10.7270/Q2V40VBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50314759 (2-(methylthio)-3-(phenylsulfonyl)-7,8-dihydro-6H-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.458 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]LSD from 5HT6 receptor in humanHeLa cells after 120 mins | Bioorg Med Chem Lett 20: 2133-6 (2010) Article DOI: 10.1016/j.bmcl.2010.02.046 BindingDB Entry DOI: 10.7270/Q2V40VBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50314760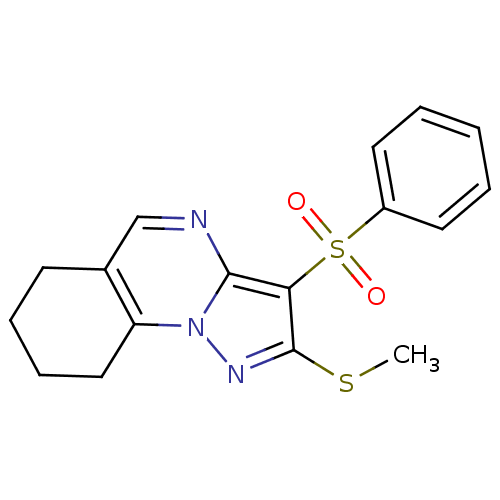 (2-(methylthio)-3-(phenylsulfonyl)-6,7,8,9-tetrahyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.549 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]LSD from 5HT6 receptor in humanHeLa cells after 120 mins | Bioorg Med Chem Lett 20: 2133-6 (2010) Article DOI: 10.1016/j.bmcl.2010.02.046 BindingDB Entry DOI: 10.7270/Q2V40VBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50314763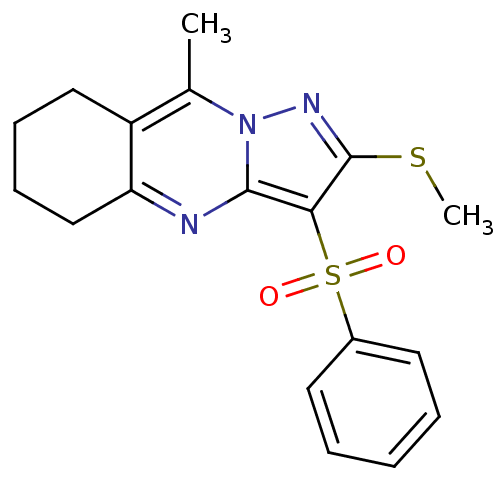 (9-methyl-2-(methylthio)-3-(phenylsulfonyl)-5,6,7,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]LSD from 5HT6 receptor in humanHeLa cells after 120 mins | Bioorg Med Chem Lett 20: 2133-6 (2010) Article DOI: 10.1016/j.bmcl.2010.02.046 BindingDB Entry DOI: 10.7270/Q2V40VBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50314761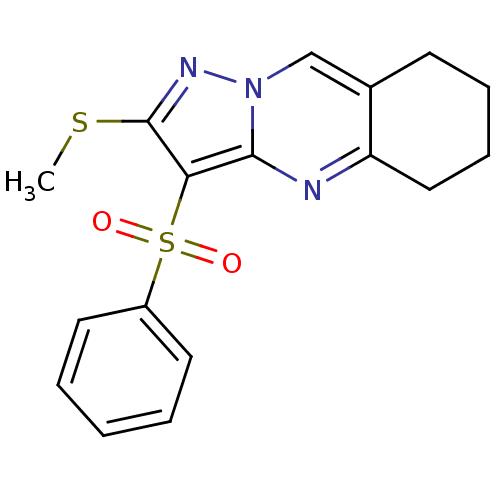 (2-Methylsulfanyl-3-phenylsulfonyl-5,6,7,8-tetrahyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]LSD from 5HT6 receptor in humanHeLa cells after 120 mins | Bioorg Med Chem Lett 20: 2133-6 (2010) Article DOI: 10.1016/j.bmcl.2010.02.046 BindingDB Entry DOI: 10.7270/Q2V40VBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50314759 (2-(methylthio)-3-(phenylsulfonyl)-7,8-dihydro-6H-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human 5HT6 receptor in HEK293 cells assessed as inhibition of serotonin-induced cAMP accumulation pretreated for 15 mins befor... | Bioorg Med Chem Lett 20: 2133-6 (2010) Article DOI: 10.1016/j.bmcl.2010.02.046 BindingDB Entry DOI: 10.7270/Q2V40VBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50314760 (2-(methylthio)-3-(phenylsulfonyl)-6,7,8,9-tetrahyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human 5HT6 receptor in HEK293 cells assessed as inhibition of serotonin-induced cAMP accumulation pretreated for 15 mins befor... | Bioorg Med Chem Lett 20: 2133-6 (2010) Article DOI: 10.1016/j.bmcl.2010.02.046 BindingDB Entry DOI: 10.7270/Q2V40VBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50314763 (9-methyl-2-(methylthio)-3-(phenylsulfonyl)-5,6,7,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human 5HT6 receptor in HEK293 cells assessed as inhibition of serotonin-induced cAMP accumulation pretreated for 15 mins befor... | Bioorg Med Chem Lett 20: 2133-6 (2010) Article DOI: 10.1016/j.bmcl.2010.02.046 BindingDB Entry DOI: 10.7270/Q2V40VBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50314762 (5-methyl-2-(methylthio)-3-(phenylsulfonyl)-6,7,8,9...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human 5HT6 receptor in HEK293 cells assessed as inhibition of serotonin-induced cAMP accumulation pretreated for 15 mins befor... | Bioorg Med Chem Lett 20: 2133-6 (2010) Article DOI: 10.1016/j.bmcl.2010.02.046 BindingDB Entry DOI: 10.7270/Q2V40VBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50314761 (2-Methylsulfanyl-3-phenylsulfonyl-5,6,7,8-tetrahyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 98.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human 5HT6 receptor in HEK293 cells assessed as inhibition of serotonin-induced cAMP accumulation pretreated for 15 mins befor... | Bioorg Med Chem Lett 20: 2133-6 (2010) Article DOI: 10.1016/j.bmcl.2010.02.046 BindingDB Entry DOI: 10.7270/Q2V40VBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50314759 (2-(methylthio)-3-(phenylsulfonyl)-7,8-dihydro-6H-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 118 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human 5HT2B receptor in HEK293 cells assessed as inhibition of alphaME-5-HT-induced cAMP accumulation pretreated for 15 secs b... | Bioorg Med Chem Lett 20: 2133-6 (2010) Article DOI: 10.1016/j.bmcl.2010.02.046 BindingDB Entry DOI: 10.7270/Q2V40VBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50314763 (9-methyl-2-(methylthio)-3-(phenylsulfonyl)-5,6,7,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human 5HT2B receptor in HEK293 cells assessed as inhibition of alphaME-5-HT-induced cAMP accumulation pretreated for 15 secs b... | Bioorg Med Chem Lett 20: 2133-6 (2010) Article DOI: 10.1016/j.bmcl.2010.02.046 BindingDB Entry DOI: 10.7270/Q2V40VBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50314762 (5-methyl-2-(methylthio)-3-(phenylsulfonyl)-6,7,8,9...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 161 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human 5HT2B receptor in HEK293 cells assessed as inhibition of alphaME-5-HT-induced cAMP accumulation pretreated for 15 secs b... | Bioorg Med Chem Lett 20: 2133-6 (2010) Article DOI: 10.1016/j.bmcl.2010.02.046 BindingDB Entry DOI: 10.7270/Q2V40VBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50314760 (2-(methylthio)-3-(phenylsulfonyl)-6,7,8,9-tetrahyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 411 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human 5HT2B receptor in HEK293 cells assessed as inhibition of alphaME-5-HT-induced cAMP accumulation pretreated for 15 secs b... | Bioorg Med Chem Lett 20: 2133-6 (2010) Article DOI: 10.1016/j.bmcl.2010.02.046 BindingDB Entry DOI: 10.7270/Q2V40VBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50314762 (5-methyl-2-(methylthio)-3-(phenylsulfonyl)-6,7,8,9...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant ERG by patch clamp assay | Bioorg Med Chem Lett 20: 2133-6 (2010) Article DOI: 10.1016/j.bmcl.2010.02.046 BindingDB Entry DOI: 10.7270/Q2V40VBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||