 Found 95 hits of Enzyme Inhibition Constant Data
Found 95 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328523
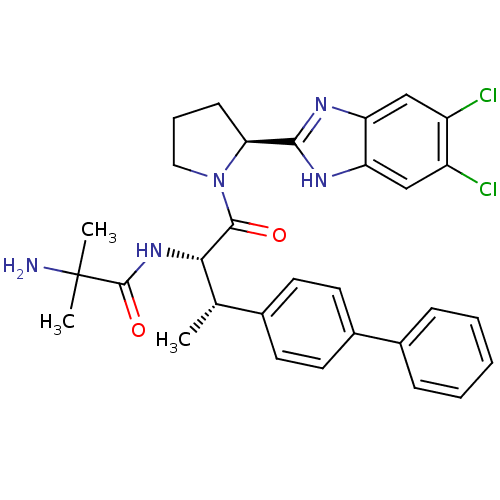
(2-Amino-N-{(2S,3S)-3-(biphenyl-4-yl)-1-[(2S)-2-(5,...)Show SMILES C[C@H]([C@H](NC(=O)C(C)(C)N)C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1)c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C31H33Cl2N5O2/c1-18(19-11-13-21(14-12-19)20-8-5-4-6-9-20)27(37-30(40)31(2,3)34)29(39)38-15-7-10-26(38)28-35-24-16-22(32)23(33)17-25(24)36-28/h4-6,8-9,11-14,16-18,26-27H,7,10,15,34H2,1-3H3,(H,35,36)(H,37,40)/t18-,26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328523

(2-Amino-N-{(2S,3S)-3-(biphenyl-4-yl)-1-[(2S)-2-(5,...)Show SMILES C[C@H]([C@H](NC(=O)C(C)(C)N)C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1)c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C31H33Cl2N5O2/c1-18(19-11-13-21(14-12-19)20-8-5-4-6-9-20)27(37-30(40)31(2,3)34)29(39)38-15-7-10-26(38)28-35-24-16-22(32)23(33)17-25(24)36-28/h4-6,8-9,11-14,16-18,26-27H,7,10,15,34H2,1-3H3,(H,35,36)(H,37,40)/t18-,26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET in presence of 1% mouse serum albumin |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328539

(2-Amino-N-{(2S)-3-(biphenyl-4-yl)-1-[(2S)-2-(5-ter...)Show SMILES CC(C)(C)c1cnc([nH]1)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)C(C)(C)N |r| Show InChI InChI=1S/C30H39N5O2/c1-29(2,3)25-19-32-26(34-25)24-12-9-17-35(24)27(36)23(33-28(37)30(4,5)31)18-20-13-15-22(16-14-20)21-10-7-6-8-11-21/h6-8,10-11,13-16,19,23-24H,9,12,17-18,31H2,1-5H3,(H,32,34)(H,33,37)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET in presence of 1% mouse serum albumin |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50328523

(2-Amino-N-{(2S,3S)-3-(biphenyl-4-yl)-1-[(2S)-2-(5,...)Show SMILES C[C@H]([C@H](NC(=O)C(C)(C)N)C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1)c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C31H33Cl2N5O2/c1-18(19-11-13-21(14-12-19)20-8-5-4-6-9-20)27(37-30(40)31(2,3)34)29(39)38-15-7-10-26(38)28-35-24-16-22(32)23(33)17-25(24)36-28/h4-6,8-9,11-14,16-18,26-27H,7,10,15,34H2,1-3H3,(H,35,36)(H,37,40)/t18-,26-,27-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328527
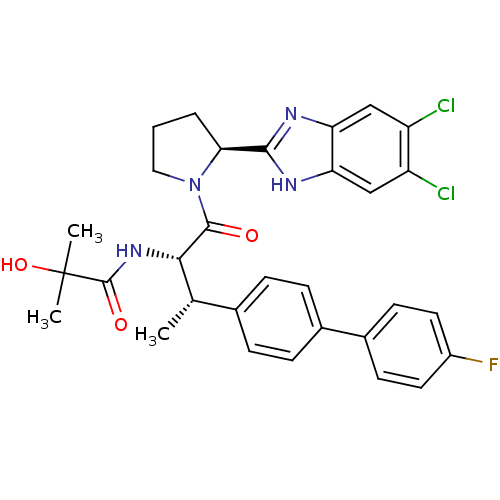
(CHEMBL1259208 | N-[(2S,3S)-1-[(2S)-2-(5,6-Dichloro...)Show SMILES C[C@H]([C@H](NC(=O)C(C)(C)O)C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1)c1ccc(cc1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C31H31Cl2FN4O3/c1-17(18-6-8-19(9-7-18)20-10-12-21(34)13-11-20)27(37-30(40)31(2,3)41)29(39)38-14-4-5-26(38)28-35-24-15-22(32)23(33)16-25(24)36-28/h6-13,15-17,26-27,41H,4-5,14H2,1-3H3,(H,35,36)(H,37,40)/t17-,26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET in presence of 1% mouse serum albumin |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328527

(CHEMBL1259208 | N-[(2S,3S)-1-[(2S)-2-(5,6-Dichloro...)Show SMILES C[C@H]([C@H](NC(=O)C(C)(C)O)C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1)c1ccc(cc1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C31H31Cl2FN4O3/c1-17(18-6-8-19(9-7-18)20-10-12-21(34)13-11-20)27(37-30(40)31(2,3)41)29(39)38-14-4-5-26(38)28-35-24-15-22(32)23(33)16-25(24)36-28/h6-13,15-17,26-27,41H,4-5,14H2,1-3H3,(H,35,36)(H,37,40)/t17-,26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328537

(3-Amino-N-{(2S)-3-(biphenyl-4-yl)-1-[(2S)-2-(5,6-d...)Show SMILES CC(C)(CN)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C31H33Cl2N5O2/c1-31(2,18-34)30(40)37-26(15-19-10-12-21(13-11-19)20-7-4-3-5-8-20)29(39)38-14-6-9-27(38)28-35-24-16-22(32)23(33)17-25(24)36-28/h3-5,7-8,10-13,16-17,26-27H,6,9,14-15,18,34H2,1-2H3,(H,35,36)(H,37,40)/t26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328522

(CHEMBL1259179 | N-{(2S)-3-(Biphenyl-4-yl)-1-[(2S)-...)Show SMILES CC(C)(C)c1cnc([nH]1)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)C(C)(C)O |r| Show InChI InChI=1S/C30H38N4O3/c1-29(2,3)25-19-31-26(33-25)24-12-9-17-34(24)27(35)23(32-28(36)30(4,5)37)18-20-13-15-22(16-14-20)21-10-7-6-8-11-21/h6-8,10-11,13-16,19,23-24,37H,9,12,17-18H2,1-5H3,(H,31,33)(H,32,36)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET in presence of 1% mouse serum albumin |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328534
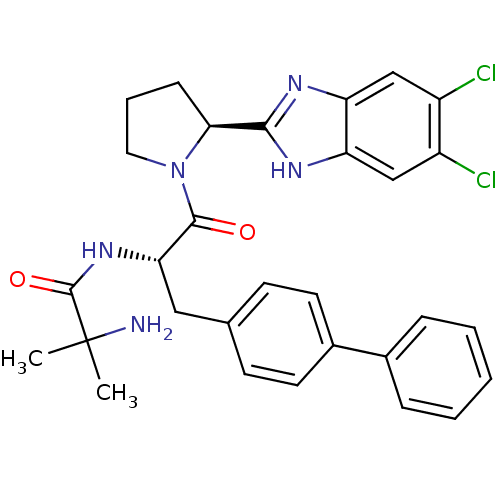
(2-Amino-N-{(2S)-3-(biphenyl-4-yl)-1-[(2S)-2-(5,6-d...)Show SMILES CC(C)(N)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C30H31Cl2N5O2/c1-30(2,33)29(39)36-25(15-18-10-12-20(13-11-18)19-7-4-3-5-8-19)28(38)37-14-6-9-26(37)27-34-23-16-21(31)22(32)17-24(23)35-27/h3-5,7-8,10-13,16-17,25-26H,6,9,14-15,33H2,1-2H3,(H,34,35)(H,36,39)/t25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328522

(CHEMBL1259179 | N-{(2S)-3-(Biphenyl-4-yl)-1-[(2S)-...)Show SMILES CC(C)(C)c1cnc([nH]1)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)C(C)(C)O |r| Show InChI InChI=1S/C30H38N4O3/c1-29(2,3)25-19-31-26(33-25)24-12-9-17-34(24)27(35)23(32-28(36)30(4,5)37)18-20-13-15-22(16-14-20)21-10-7-6-8-11-21/h6-8,10-11,13-16,19,23-24,37H,9,12,17-18H2,1-5H3,(H,31,33)(H,32,36)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328524

(5,6-Dichloro-2-{(2S)-1-[2-methylalanyl-(betaS)-bet...)Show SMILES C[C@H]([C@H](NC(=O)C(C)(C)N)C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1)c1ccccc1 |r| Show InChI InChI=1S/C25H29Cl2N5O2/c1-14(15-8-5-4-6-9-15)21(31-24(34)25(2,3)28)23(33)32-11-7-10-20(32)22-29-18-12-16(26)17(27)13-19(18)30-22/h4-6,8-9,12-14,20-21H,7,10-11,28H2,1-3H3,(H,29,30)(H,31,34)/t14-,20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328538

(3-Amino-N-{(2S)-3-(biphenyl-4-yl)-1-[(2S)-2-(5,6-d...)Show SMILES CC(C)(N)CC(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C31H33Cl2N5O2/c1-31(2,34)18-28(39)35-26(15-19-10-12-21(13-11-19)20-7-4-3-5-8-20)30(40)38-14-6-9-27(38)29-36-24-16-22(32)23(33)17-25(24)37-29/h3-5,7-8,10-13,16-17,26-27H,6,9,14-15,18,34H2,1-2H3,(H,35,39)(H,36,37)/t26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328528
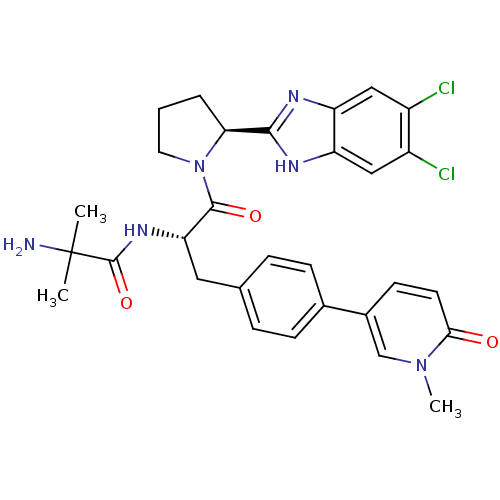
(5,6-Dichloro-2-{(2S)-1-[2-methylalanyl-4-(1-methyl...)Show SMILES Cn1cc(ccc1=O)-c1ccc(C[C@H](NC(=O)C(C)(C)N)C(=O)N2CCC[C@H]2c2nc3cc(Cl)c(Cl)cc3[nH]2)cc1 |r| Show InChI InChI=1S/C30H32Cl2N6O3/c1-30(2,33)29(41)36-24(13-17-6-8-18(9-7-17)19-10-11-26(39)37(3)16-19)28(40)38-12-4-5-25(38)27-34-22-14-20(31)21(32)15-23(22)35-27/h6-11,14-16,24-25H,4-5,12-13,33H2,1-3H3,(H,34,35)(H,36,41)/t24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET in presence of 1% mouse serum albumin |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328539

(2-Amino-N-{(2S)-3-(biphenyl-4-yl)-1-[(2S)-2-(5-ter...)Show SMILES CC(C)(C)c1cnc([nH]1)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)C(C)(C)N |r| Show InChI InChI=1S/C30H39N5O2/c1-29(2,3)25-19-32-26(34-25)24-12-9-17-35(24)27(36)23(33-28(37)30(4,5)31)18-20-13-15-22(16-14-20)21-10-7-6-8-11-21/h6-8,10-11,13-16,19,23-24H,9,12,17-18,31H2,1-5H3,(H,32,34)(H,33,37)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328528

(5,6-Dichloro-2-{(2S)-1-[2-methylalanyl-4-(1-methyl...)Show SMILES Cn1cc(ccc1=O)-c1ccc(C[C@H](NC(=O)C(C)(C)N)C(=O)N2CCC[C@H]2c2nc3cc(Cl)c(Cl)cc3[nH]2)cc1 |r| Show InChI InChI=1S/C30H32Cl2N6O3/c1-30(2,33)29(41)36-24(13-17-6-8-18(9-7-17)19-10-11-26(39)37(3)16-19)28(40)38-12-4-5-25(38)27-34-22-14-20(31)21(32)15-23(22)35-27/h6-11,14-16,24-25H,4-5,12-13,33H2,1-3H3,(H,34,35)(H,36,41)/t24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50328523

(2-Amino-N-{(2S,3S)-3-(biphenyl-4-yl)-1-[(2S)-2-(5,...)Show SMILES C[C@H]([C@H](NC(=O)C(C)(C)N)C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1)c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C31H33Cl2N5O2/c1-18(19-11-13-21(14-12-19)20-8-5-4-6-9-20)27(37-30(40)31(2,3)34)29(39)38-15-7-10-26(38)28-35-24-16-22(32)23(33)17-25(24)36-28/h4-6,8-9,11-14,16-18,26-27H,7,10,15,34H2,1-3H3,(H,35,36)(H,37,40)/t18-,26-,27-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP by FRET in presence of 1% mouse serum albumin |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328536
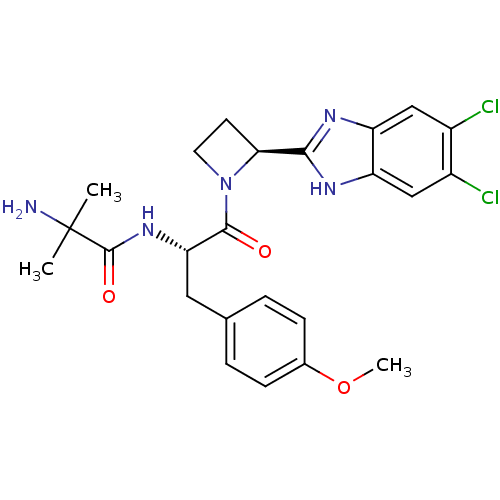
(5,6-Dichloro-2-[(2S)-1-(2-methylalanyl-O-methyl-L-...)Show SMILES COc1ccc(C[C@H](NC(=O)C(C)(C)N)C(=O)N2CC[C@H]2c2nc3cc(Cl)c(Cl)cc3[nH]2)cc1 |r| Show InChI InChI=1S/C24H27Cl2N5O3/c1-24(2,27)23(33)30-19(10-13-4-6-14(34-3)7-5-13)22(32)31-9-8-20(31)21-28-17-11-15(25)16(26)12-18(17)29-21/h4-7,11-12,19-20H,8-10,27H2,1-3H3,(H,28,29)(H,30,33)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328524

(5,6-Dichloro-2-{(2S)-1-[2-methylalanyl-(betaS)-bet...)Show SMILES C[C@H]([C@H](NC(=O)C(C)(C)N)C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1)c1ccccc1 |r| Show InChI InChI=1S/C25H29Cl2N5O2/c1-14(15-8-5-4-6-9-15)21(31-24(34)25(2,3)28)23(33)32-11-7-10-20(32)22-29-18-12-16(26)17(27)13-19(18)30-22/h4-6,8-9,12-14,20-21H,7,10-11,28H2,1-3H3,(H,29,30)(H,31,34)/t14-,20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET in presence of 1% mouse serum albumin |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328529
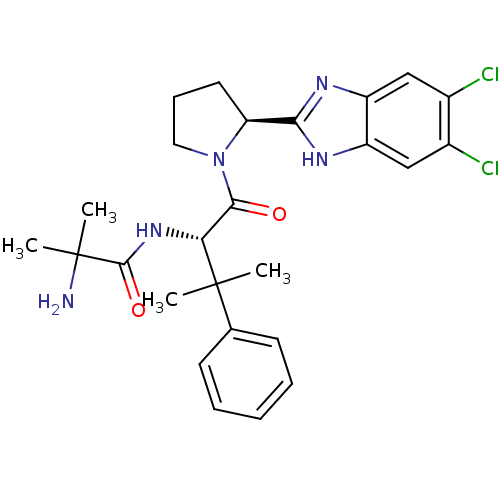
(5,6-Dichloro-2-[(2S)-1-(2-methylalanyl-beta,beta-d...)Show SMILES CC(C)(N)C(=O)N[C@H](C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1)C(C)(C)c1ccccc1 |r| Show InChI InChI=1S/C26H31Cl2N5O2/c1-25(2,15-9-6-5-7-10-15)21(32-24(35)26(3,4)29)23(34)33-12-8-11-20(33)22-30-18-13-16(27)17(28)14-19(18)31-22/h5-7,9-10,13-14,20-21H,8,11-12,29H2,1-4H3,(H,30,31)(H,32,35)/t20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50328522

(CHEMBL1259179 | N-{(2S)-3-(Biphenyl-4-yl)-1-[(2S)-...)Show SMILES CC(C)(C)c1cnc([nH]1)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)C(C)(C)O |r| Show InChI InChI=1S/C30H38N4O3/c1-29(2,3)25-19-31-26(33-25)24-12-9-17-34(24)27(35)23(32-28(36)30(4,5)37)18-20-13-15-22(16-14-20)21-10-7-6-8-11-21/h6-8,10-11,13-16,19,23-24,37H,9,12,17-18H2,1-5H3,(H,31,33)(H,32,36)/t23-,24-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP by FRET in presence of 1% mouse serum albumin |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50328534

(2-Amino-N-{(2S)-3-(biphenyl-4-yl)-1-[(2S)-2-(5,6-d...)Show SMILES CC(C)(N)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C30H31Cl2N5O2/c1-30(2,33)29(39)36-25(15-18-10-12-20(13-11-18)19-7-4-3-5-8-19)28(38)37-14-6-9-26(37)27-34-23-16-21(31)22(32)17-24(23)35-27/h3-5,7-8,10-13,16-17,25-26H,6,9,14-15,33H2,1-2H3,(H,34,35)(H,36,39)/t25-,26-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50328522

(CHEMBL1259179 | N-{(2S)-3-(Biphenyl-4-yl)-1-[(2S)-...)Show SMILES CC(C)(C)c1cnc([nH]1)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)C(C)(C)O |r| Show InChI InChI=1S/C30H38N4O3/c1-29(2,3)25-19-31-26(33-25)24-12-9-17-34(24)27(35)23(32-28(36)30(4,5)37)18-20-13-15-22(16-14-20)21-10-7-6-8-11-21/h6-8,10-11,13-16,19,23-24,37H,9,12,17-18H2,1-5H3,(H,31,33)(H,32,36)/t23-,24-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50328539

(2-Amino-N-{(2S)-3-(biphenyl-4-yl)-1-[(2S)-2-(5-ter...)Show SMILES CC(C)(C)c1cnc([nH]1)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)C(C)(C)N |r| Show InChI InChI=1S/C30H39N5O2/c1-29(2,3)25-19-32-26(34-25)24-12-9-17-35(24)27(36)23(33-28(37)30(4,5)31)18-20-13-15-22(16-14-20)21-10-7-6-8-11-21/h6-8,10-11,13-16,19,23-24H,9,12,17-18,31H2,1-5H3,(H,32,34)(H,33,37)/t23-,24-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP by FRET in presence of 1% mouse serum albumin |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328525
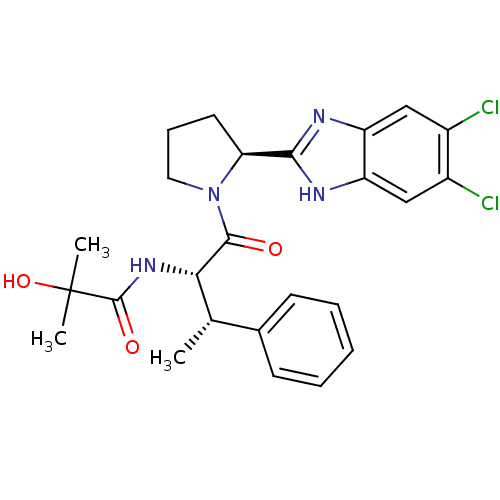
(CHEMBL1259221 | N-{(2S,3S)-1-[(2S)-2-(5,6-Dichloro...)Show SMILES C[C@H]([C@H](NC(=O)C(C)(C)O)C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1)c1ccccc1 |r| Show InChI InChI=1S/C25H28Cl2N4O3/c1-14(15-8-5-4-6-9-15)21(30-24(33)25(2,3)34)23(32)31-11-7-10-20(31)22-28-18-12-16(26)17(27)13-19(18)29-22/h4-6,8-9,12-14,20-21,34H,7,10-11H2,1-3H3,(H,28,29)(H,30,33)/t14-,20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328583
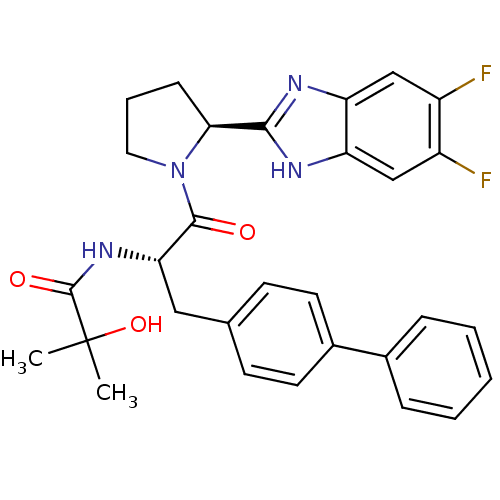
(CHEMBL1259135 | N-{(2S)-3-(Biphenyl-4-yl)-1-[(2S)-...)Show SMILES CC(C)(O)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(=O)N1CCC[C@H]1c1nc2cc(F)c(F)cc2[nH]1 |r| Show InChI InChI=1S/C30H30F2N4O3/c1-30(2,39)29(38)35-25(15-18-10-12-20(13-11-18)19-7-4-3-5-8-19)28(37)36-14-6-9-26(36)27-33-23-16-21(31)22(32)17-24(23)34-27/h3-5,7-8,10-13,16-17,25-26,39H,6,9,14-15H2,1-2H3,(H,33,34)(H,35,38)/t25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328532
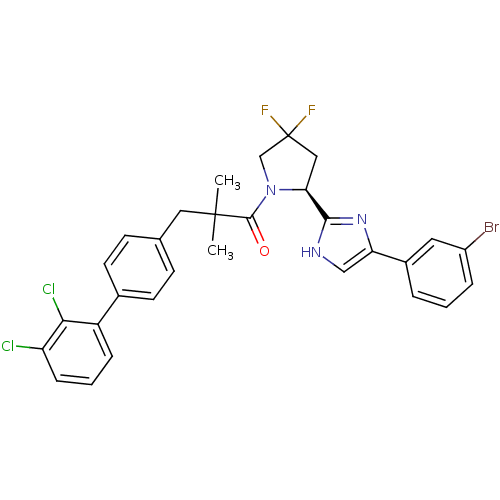
((S)-1-(2-(5-(3-bromophenyl)-1H-imidazol-2-yl)-4,4-...)Show SMILES CC(C)(Cc1ccc(cc1)-c1cccc(Cl)c1Cl)C(=O)N1CC(F)(F)C[C@H]1c1nc(c[nH]1)-c1cccc(Br)c1 |r| Show InChI InChI=1S/C30H26BrCl2F2N3O/c1-29(2,14-18-9-11-19(12-10-18)22-7-4-8-23(32)26(22)33)28(39)38-17-30(34,35)15-25(38)27-36-16-24(37-27)20-5-3-6-21(31)13-20/h3-13,16,25H,14-15,17H2,1-2H3,(H,36,37)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328582
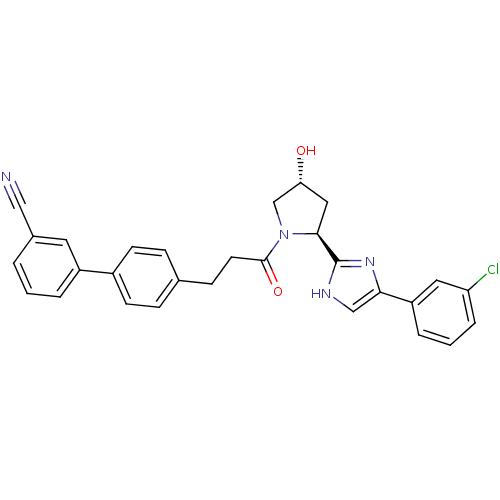
(4'-(3-{(2S,4R)-2-[5-(3-Chlorophenyl)-1H-imidazol-2...)Show SMILES O[C@@H]1C[C@H](N(C1)C(=O)CCc1ccc(cc1)-c1cccc(c1)C#N)c1nc(c[nH]1)-c1cccc(Cl)c1 |r| Show InChI InChI=1S/C29H25ClN4O2/c30-24-6-2-5-23(14-24)26-17-32-29(33-26)27-15-25(35)18-34(27)28(36)12-9-19-7-10-21(11-8-19)22-4-1-3-20(13-22)16-31/h1-8,10-11,13-14,17,25,27,35H,9,12,15,18H2,(H,32,33)/t25-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50328525

(CHEMBL1259221 | N-{(2S,3S)-1-[(2S)-2-(5,6-Dichloro...)Show SMILES C[C@H]([C@H](NC(=O)C(C)(C)O)C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1)c1ccccc1 |r| Show InChI InChI=1S/C25H28Cl2N4O3/c1-14(15-8-5-4-6-9-15)21(30-24(33)25(2,3)34)23(32)31-11-7-10-20(31)22-28-18-12-16(26)17(27)13-19(18)29-22/h4-6,8-9,12-14,20-21,34H,7,10-11H2,1-3H3,(H,28,29)(H,30,33)/t14-,20-,21-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50328524

(5,6-Dichloro-2-{(2S)-1-[2-methylalanyl-(betaS)-bet...)Show SMILES C[C@H]([C@H](NC(=O)C(C)(C)N)C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1)c1ccccc1 |r| Show InChI InChI=1S/C25H29Cl2N5O2/c1-14(15-8-5-4-6-9-15)21(31-24(34)25(2,3)28)23(33)32-11-7-10-20(32)22-29-18-12-16(26)17(27)13-19(18)30-22/h4-6,8-9,12-14,20-21H,7,10-11,28H2,1-3H3,(H,29,30)(H,31,34)/t14-,20-,21-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328526

(5,6-Dichloro-2-{(2S)-1-[2-methylalanyl-(betaS)-bet...)Show SMILES C[C@H]([C@H](NC(=O)C(C)(C)N)C(=O)N1CC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1)c1ccccc1 |r| Show InChI InChI=1S/C24H27Cl2N5O2/c1-13(14-7-5-4-6-8-14)20(30-23(33)24(2,3)27)22(32)31-10-9-19(31)21-28-17-11-15(25)16(26)12-18(17)29-21/h4-8,11-13,19-20H,9-10,27H2,1-3H3,(H,28,29)(H,30,33)/t13-,19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328529

(5,6-Dichloro-2-[(2S)-1-(2-methylalanyl-beta,beta-d...)Show SMILES CC(C)(N)C(=O)N[C@H](C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1)C(C)(C)c1ccccc1 |r| Show InChI InChI=1S/C26H31Cl2N5O2/c1-25(2,15-9-6-5-7-10-15)21(32-24(35)26(3,4)29)23(34)33-12-8-11-20(33)22-30-18-13-16(27)17(28)14-19(18)31-22/h5-7,9-10,13-14,20-21H,8,11-12,29H2,1-4H3,(H,30,31)(H,32,35)/t20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET in presence of 1% mouse serum albumin |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50328539

(2-Amino-N-{(2S)-3-(biphenyl-4-yl)-1-[(2S)-2-(5-ter...)Show SMILES CC(C)(C)c1cnc([nH]1)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)C(C)(C)N |r| Show InChI InChI=1S/C30H39N5O2/c1-29(2,3)25-19-32-26(34-25)24-12-9-17-35(24)27(36)23(33-28(37)30(4,5)31)18-20-13-15-22(16-14-20)21-10-7-6-8-11-21/h6-8,10-11,13-16,19,23-24H,9,12,17-18,31H2,1-5H3,(H,32,34)(H,33,37)/t23-,24-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328537

(3-Amino-N-{(2S)-3-(biphenyl-4-yl)-1-[(2S)-2-(5,6-d...)Show SMILES CC(C)(CN)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C31H33Cl2N5O2/c1-31(2,18-34)30(40)37-26(15-19-10-12-21(13-11-19)20-7-4-3-5-8-20)29(39)38-14-6-9-27(38)28-35-24-16-22(32)23(33)17-25(24)36-28/h3-5,7-8,10-13,16-17,26-27H,6,9,14-15,18,34H2,1-2H3,(H,35,36)(H,37,40)/t26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET in presence of 1% mouse serum albumin |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328559
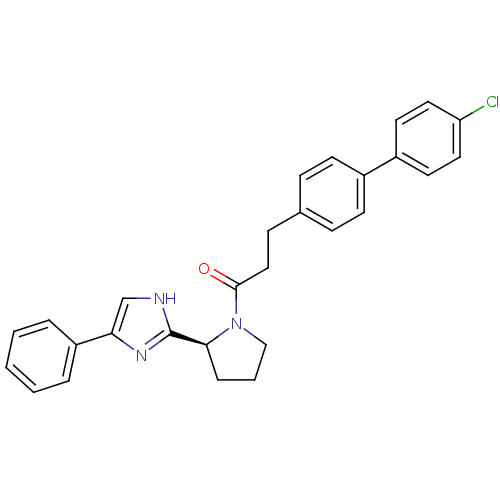
(3-(4'-Chlorobiphenyl-4-yl)-1-[(2S)-2-(4-phenyl-1H-...)Show SMILES Clc1ccc(cc1)-c1ccc(CCC(=O)N2CCC[C@H]2c2nc(c[nH]2)-c2ccccc2)cc1 |r| Show InChI InChI=1S/C28H26ClN3O/c29-24-15-13-22(14-16-24)21-11-8-20(9-12-21)10-17-27(33)32-18-4-7-26(32)28-30-19-25(31-28)23-5-2-1-3-6-23/h1-3,5-6,8-9,11-16,19,26H,4,7,10,17-18H2,(H,30,31)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328535
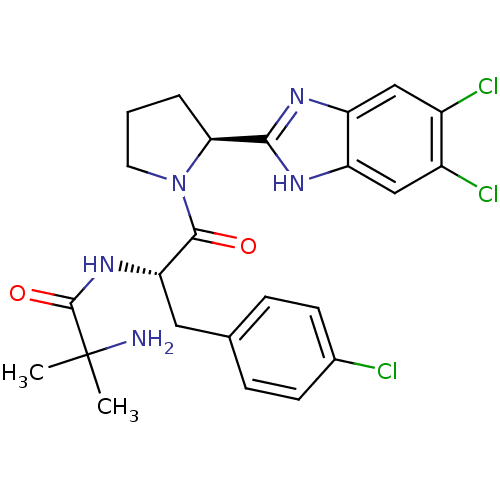
(5,6-Dichloro-2-[(2S)-1-(2-methylalanyl-4-chloro-L-...)Show SMILES CC(C)(N)C(=O)N[C@@H](Cc1ccc(Cl)cc1)C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C24H26Cl3N5O2/c1-24(2,28)23(34)31-19(10-13-5-7-14(25)8-6-13)22(33)32-9-3-4-20(32)21-29-17-11-15(26)16(27)12-18(17)30-21/h5-8,11-12,19-20H,3-4,9-10,28H2,1-2H3,(H,29,30)(H,31,34)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328557
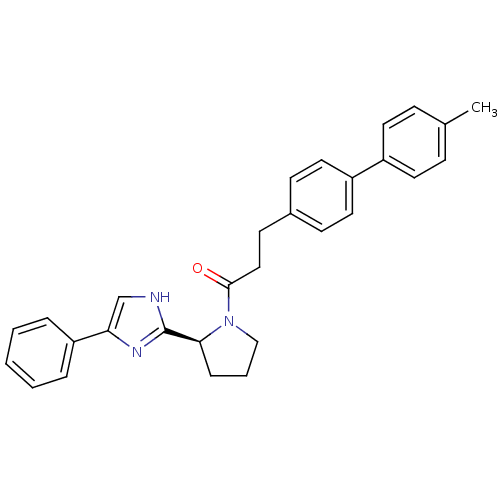
(3-(4'-Methylbiphenyl-4-yl)-1-[(2S)-2-(4-phenyl-1H-...)Show SMILES Cc1ccc(cc1)-c1ccc(CCC(=O)N2CCC[C@H]2c2nc(c[nH]2)-c2ccccc2)cc1 |r| Show InChI InChI=1S/C29H29N3O/c1-21-9-14-23(15-10-21)24-16-11-22(12-17-24)13-18-28(33)32-19-5-8-27(32)29-30-20-26(31-29)25-6-3-2-4-7-25/h2-4,6-7,9-12,14-17,20,27H,5,8,13,18-19H2,1H3,(H,30,31)/t27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328525

(CHEMBL1259221 | N-{(2S,3S)-1-[(2S)-2-(5,6-Dichloro...)Show SMILES C[C@H]([C@H](NC(=O)C(C)(C)O)C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1)c1ccccc1 |r| Show InChI InChI=1S/C25H28Cl2N4O3/c1-14(15-8-5-4-6-9-15)21(30-24(33)25(2,3)34)23(32)31-11-7-10-20(31)22-28-18-12-16(26)17(27)13-19(18)29-22/h4-6,8-9,12-14,20-21,34H,7,10-11H2,1-3H3,(H,28,29)(H,30,33)/t14-,20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET in presence of 1% mouse serum albumin |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50328524

(5,6-Dichloro-2-{(2S)-1-[2-methylalanyl-(betaS)-bet...)Show SMILES C[C@H]([C@H](NC(=O)C(C)(C)N)C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1)c1ccccc1 |r| Show InChI InChI=1S/C25H29Cl2N5O2/c1-14(15-8-5-4-6-9-15)21(31-24(34)25(2,3)28)23(33)32-11-7-10-20(32)22-29-18-12-16(26)17(27)13-19(18)30-22/h4-6,8-9,12-14,20-21H,7,10-11,28H2,1-3H3,(H,29,30)(H,31,34)/t14-,20-,21-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP by FRET in presence of 1% mouse serum albumin |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328526

(5,6-Dichloro-2-{(2S)-1-[2-methylalanyl-(betaS)-bet...)Show SMILES C[C@H]([C@H](NC(=O)C(C)(C)N)C(=O)N1CC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1)c1ccccc1 |r| Show InChI InChI=1S/C24H27Cl2N5O2/c1-13(14-7-5-4-6-8-14)20(30-23(33)24(2,3)27)22(32)31-10-9-19(31)21-28-17-11-15(25)16(26)12-18(17)29-21/h4-8,11-13,19-20H,9-10,27H2,1-3H3,(H,28,29)(H,30,33)/t13-,19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET in presence of 1% mouse serum albumin |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328550
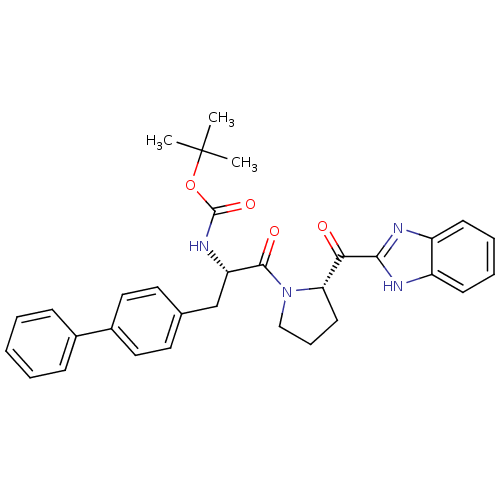
(CHEMBL1259218 | tert-Butyl{(2S)-3-(Biphenyl-4-yl)-...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(=O)N1CCC[C@H]1C(=O)c1nc2ccccc2[nH]1 |r| Show InChI InChI=1S/C32H34N4O4/c1-32(2,3)40-31(39)35-26(20-21-15-17-23(18-16-21)22-10-5-4-6-11-22)30(38)36-19-9-14-27(36)28(37)29-33-24-12-7-8-13-25(24)34-29/h4-8,10-13,15-18,26-27H,9,14,19-20H2,1-3H3,(H,33,34)(H,35,39)/t26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328538

(3-Amino-N-{(2S)-3-(biphenyl-4-yl)-1-[(2S)-2-(5,6-d...)Show SMILES CC(C)(N)CC(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C31H33Cl2N5O2/c1-31(2,34)18-28(39)35-26(15-19-10-12-21(13-11-19)20-7-4-3-5-8-20)30(40)38-14-6-9-27(38)29-36-24-16-22(32)23(33)17-25(24)37-29/h3-5,7-8,10-13,16-17,26-27H,6,9,14-15,18,34H2,1-2H3,(H,35,39)(H,36,37)/t26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET in presence of 1% mouse serum albumin |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328560

(3-(4'-tert-Butylbiphenyl-4-yl)-1-[(2S)-2-(4-phenyl...)Show SMILES CC(C)(C)c1ccc(cc1)-c1ccc(CCC(=O)N2CCC[C@H]2c2nc(c[nH]2)-c2ccccc2)cc1 |r| Show InChI InChI=1S/C32H35N3O/c1-32(2,3)27-18-16-25(17-19-27)24-14-11-23(12-15-24)13-20-30(36)35-21-7-10-29(35)31-33-22-28(34-31)26-8-5-4-6-9-26/h4-6,8-9,11-12,14-19,22,29H,7,10,13,20-21H2,1-3H3,(H,33,34)/t29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328561

(3-(4'-Chloro-3'-fluorobiphenyl-4-yl)-1-[(2S)-2-(4-...)Show SMILES Fc1cc(ccc1Cl)-c1ccc(CCC(=O)N2CCC[C@H]2c2nc(c[nH]2)-c2ccccc2)cc1 |r| Show InChI InChI=1S/C28H25ClFN3O/c29-23-14-13-22(17-24(23)30)20-11-8-19(9-12-20)10-15-27(34)33-16-4-7-26(33)28-31-18-25(32-28)21-5-2-1-3-6-21/h1-3,5-6,8-9,11-14,17-18,26H,4,7,10,15-16H2,(H,31,32)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328531
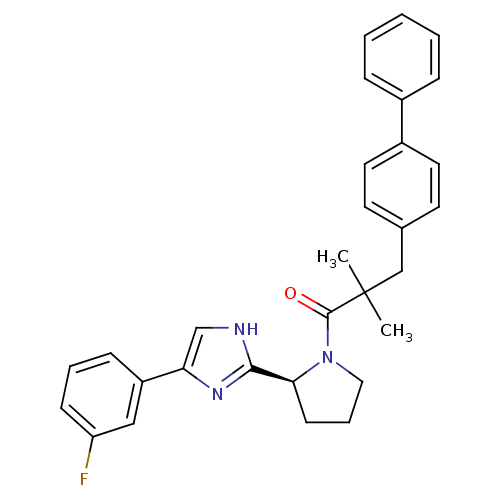
(3-(Biphenyl-4-yl)-1-{2-[5-(3-fluorophenyl)-1H-imid...)Show SMILES CC(C)(Cc1ccc(cc1)-c1ccccc1)C(=O)N1CCC[C@H]1c1nc(c[nH]1)-c1cccc(F)c1 |r| Show InChI InChI=1S/C30H30FN3O/c1-30(2,19-21-13-15-23(16-14-21)22-8-4-3-5-9-22)29(35)34-17-7-12-27(34)28-32-20-26(33-28)24-10-6-11-25(31)18-24/h3-6,8-11,13-16,18,20,27H,7,12,17,19H2,1-2H3,(H,32,33)/t27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328549
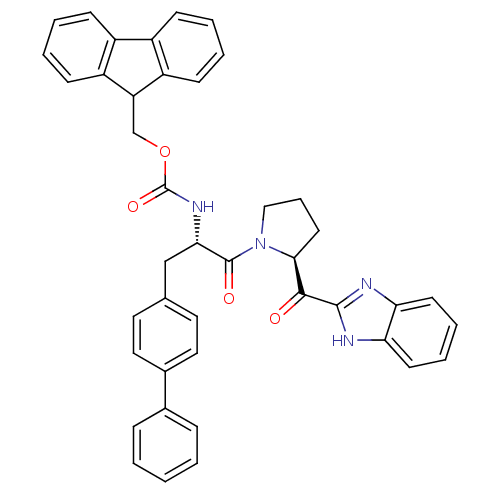
(9H-Fluoren-9-ylmethyl{(2S)-3-(Biphenyl-4-yl)-1-oxo...)Show SMILES O=C(N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(=O)N1CCC[C@H]1C(=O)c1nc2ccccc2[nH]1)OCC1c2ccccc2-c2ccccc12 |r| Show InChI InChI=1S/C42H36N4O4/c47-39(40-43-35-17-8-9-18-36(35)44-40)38-19-10-24-46(38)41(48)37(25-27-20-22-29(23-21-27)28-11-2-1-3-12-28)45-42(49)50-26-34-32-15-6-4-13-30(32)31-14-5-7-16-33(31)34/h1-9,11-18,20-23,34,37-38H,10,19,24-26H2,(H,43,44)(H,45,49)/t37-,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50328525

(CHEMBL1259221 | N-{(2S,3S)-1-[(2S)-2-(5,6-Dichloro...)Show SMILES C[C@H]([C@H](NC(=O)C(C)(C)O)C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1)c1ccccc1 |r| Show InChI InChI=1S/C25H28Cl2N4O3/c1-14(15-8-5-4-6-9-15)21(30-24(33)25(2,3)34)23(32)31-11-7-10-20(31)22-28-18-12-16(26)17(27)13-19(18)29-22/h4-6,8-9,12-14,20-21,34H,7,10-11H2,1-3H3,(H,28,29)(H,30,33)/t14-,20-,21-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP by FRET in presence of 1% mouse serum albumin |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328535

(5,6-Dichloro-2-[(2S)-1-(2-methylalanyl-4-chloro-L-...)Show SMILES CC(C)(N)C(=O)N[C@@H](Cc1ccc(Cl)cc1)C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C24H26Cl3N5O2/c1-24(2,28)23(34)31-19(10-13-5-7-14(25)8-6-13)22(33)32-9-3-4-20(32)21-29-17-11-15(26)16(27)12-18(17)30-21/h5-8,11-12,19-20H,3-4,9-10,28H2,1-2H3,(H,29,30)(H,31,34)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET in presence of 1% mouse serum albumin |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328558

(3-(2'-Chlorobiphenyl-4-yl)-1-[(2S)-2-(4-phenyl-1H-...)Show SMILES Clc1ccccc1-c1ccc(CCC(=O)N2CCC[C@H]2c2nc(c[nH]2)-c2ccccc2)cc1 |r| Show InChI InChI=1S/C28H26ClN3O/c29-24-10-5-4-9-23(24)21-15-12-20(13-16-21)14-17-27(33)32-18-6-11-26(32)28-30-19-25(31-28)22-7-2-1-3-8-22/h1-5,7-10,12-13,15-16,19,26H,6,11,14,17-18H2,(H,30,31)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 122 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328530
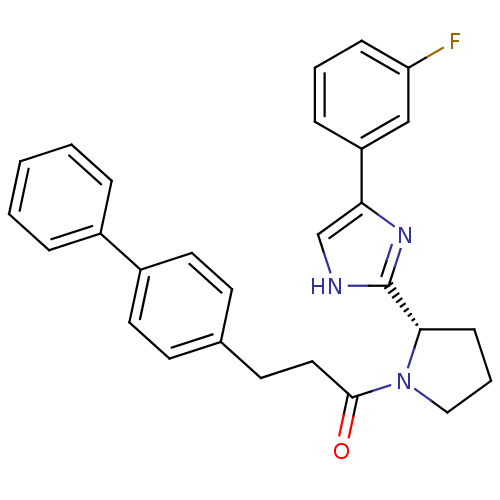
(3-(Biphenyl-4-yl)-1-{2-[5-(3-fluorophenyl)-1H-imid...)Show SMILES Fc1cccc(c1)-c1c[nH]c(n1)[C@@H]1CCCN1C(=O)CCc1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C28H26FN3O/c29-24-9-4-8-23(18-24)25-19-30-28(31-25)26-10-5-17-32(26)27(33)16-13-20-11-14-22(15-12-20)21-6-2-1-3-7-21/h1-4,6-9,11-12,14-15,18-19,26H,5,10,13,16-17H2,(H,30,31)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328554
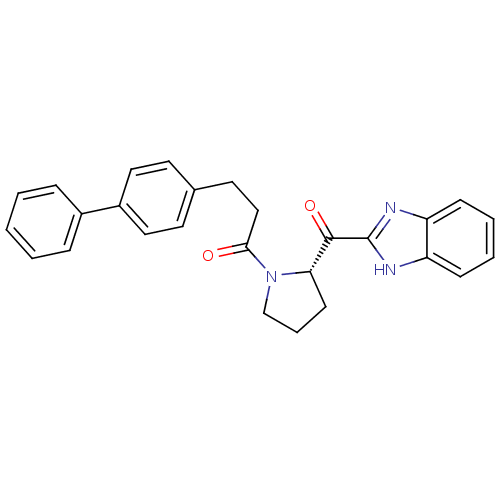
(3-(Biphenyl-4-yl)-1-[(2S)-2-(4-phenyl-1H-imidazol-...)Show SMILES O=C(CCc1ccc(cc1)-c1ccccc1)N1CCC[C@H]1C(=O)c1nc2ccccc2[nH]1 |r| Show InChI InChI=1S/C27H25N3O2/c31-25(17-14-19-12-15-21(16-13-19)20-7-2-1-3-8-20)30-18-6-11-24(30)26(32)27-28-22-9-4-5-10-23(22)29-27/h1-5,7-10,12-13,15-16,24H,6,11,14,17-18H2,(H,28,29)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 223 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328547
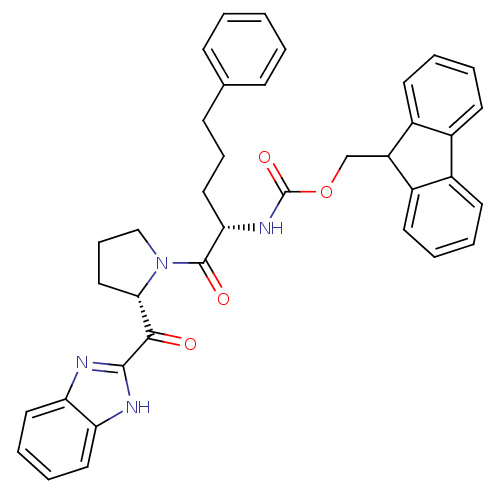
(9H-Fluoren-9-ylmethyl{(2S)-1-Oxo-5-phenyl-1-[(2S)-...)Show SMILES O=C(N[C@@H](CCCc1ccccc1)C(=O)N1CCC[C@H]1C(=O)c1nc2ccccc2[nH]1)OCC1c2ccccc2-c2ccccc12 |r| Show InChI InChI=1S/C38H36N4O4/c43-35(36-39-31-19-8-9-20-32(31)40-36)34-22-11-23-42(34)37(44)33(21-10-14-25-12-2-1-3-13-25)41-38(45)46-24-30-28-17-6-4-15-26(28)27-16-5-7-18-29(27)30/h1-9,12-13,15-20,30,33-34H,10-11,14,21-24H2,(H,39,40)(H,41,45)/t33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50328536

(5,6-Dichloro-2-[(2S)-1-(2-methylalanyl-O-methyl-L-...)Show SMILES COc1ccc(C[C@H](NC(=O)C(C)(C)N)C(=O)N2CC[C@H]2c2nc3cc(Cl)c(Cl)cc3[nH]2)cc1 |r| Show InChI InChI=1S/C24H27Cl2N5O3/c1-24(2,27)23(33)30-19(10-13-4-6-14(34-3)7-5-13)22(32)31-9-8-20(31)21-28-17-11-15(25)16(26)12-18(17)29-21/h4-7,11-12,19-20H,8-10,27H2,1-3H3,(H,28,29)(H,30,33)/t19-,20-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328541
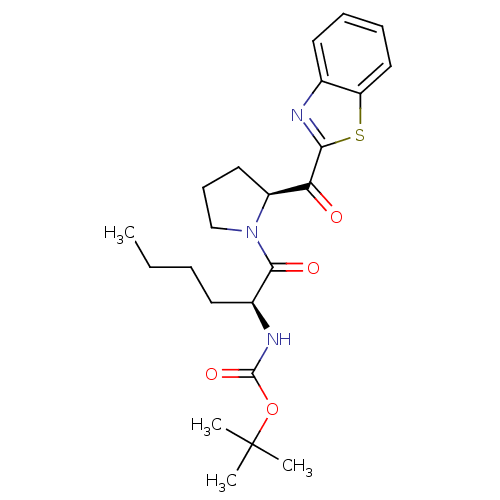
(CHEMBL1259114 | tert-butyl(S)-1-((S)-2-(benzo[d]th...)Show SMILES CCCC[C@H](NC(=O)OC(C)(C)C)C(=O)N1CCC[C@H]1C(=O)c1nc2ccccc2s1 |r| Show InChI InChI=1S/C23H31N3O4S/c1-5-6-10-16(25-22(29)30-23(2,3)4)21(28)26-14-9-12-17(26)19(27)20-24-15-11-7-8-13-18(15)31-20/h7-8,11,13,16-17H,5-6,9-10,12,14H2,1-4H3,(H,25,29)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328562
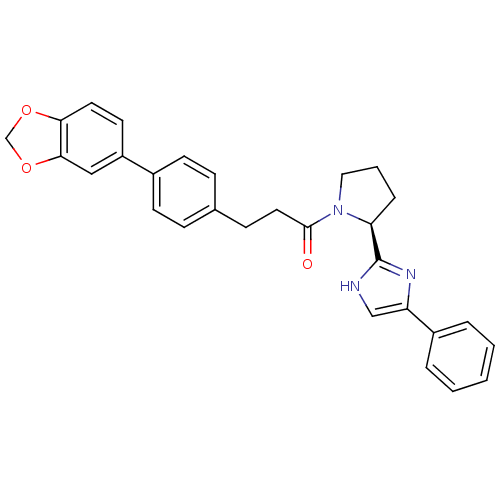
(3-[4-(1,3-Benzodioxol-5-yl)phenyl]-1-[(2S)-2-(4-ph...)Show SMILES O=C(CCc1ccc(cc1)-c1ccc2OCOc2c1)N1CCC[C@H]1c1nc(c[nH]1)-c1ccccc1 |r| Show InChI InChI=1S/C29H27N3O3/c33-28(32-16-4-7-25(32)29-30-18-24(31-29)22-5-2-1-3-6-22)15-10-20-8-11-21(12-9-20)23-13-14-26-27(17-23)35-19-34-26/h1-3,5-6,8-9,11-14,17-18,25H,4,7,10,15-16,19H2,(H,30,31)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
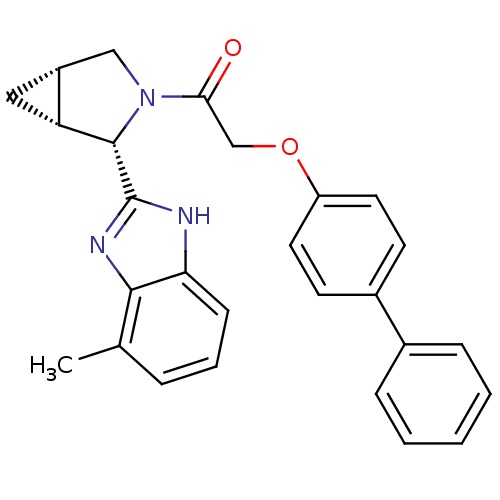
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328533
(2-(biphenyl-4-yloxy)-1-((1R,2S,5S)-2-(4-methyl-1H-...)Show SMILES Cc1cccc2[nH]c(nc12)[C@@H]1[C@@H]2C[C@@H]2CN1C(=O)COc1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C27H25N3O2/c1-17-6-5-9-23-25(17)29-27(28-23)26-22-14-20(22)15-30(26)24(31)16-32-21-12-10-19(11-13-21)18-7-3-2-4-8-18/h2-13,20,22,26H,14-16H2,1H3,(H,28,29)/t20-,22-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 308 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
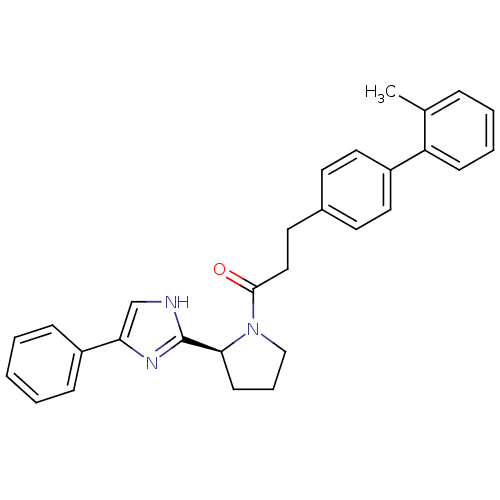
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328556
(3-(2'-Methylbiphenyl-4-yl)-1-[(2S)-2-(4-phenyl-1H-...)Show SMILES Cc1ccccc1-c1ccc(CCC(=O)N2CCC[C@H]2c2nc(c[nH]2)-c2ccccc2)cc1 |r| Show InChI InChI=1S/C29H29N3O/c1-21-8-5-6-11-25(21)23-16-13-22(14-17-23)15-18-28(33)32-19-7-12-27(32)29-30-20-26(31-29)24-9-3-2-4-10-24/h2-6,8-11,13-14,16-17,20,27H,7,12,15,18-19H2,1H3,(H,30,31)/t27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 352 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328534

(2-Amino-N-{(2S)-3-(biphenyl-4-yl)-1-[(2S)-2-(5,6-d...)Show SMILES CC(C)(N)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C30H31Cl2N5O2/c1-30(2,33)29(39)36-25(15-18-10-12-20(13-11-18)19-7-4-3-5-8-19)28(38)37-14-6-9-26(37)27-34-23-16-21(31)22(32)17-24(23)35-27/h3-5,7-8,10-13,16-17,25-26H,6,9,14-15,33H2,1-2H3,(H,34,35)(H,36,39)/t25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 356 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET in presence of 1% mouse serum albumin |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
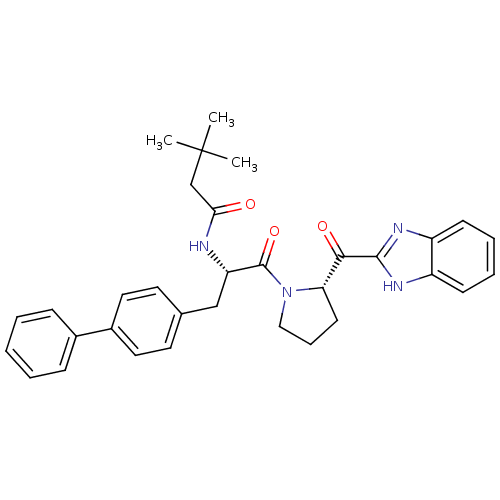
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328551
(CHEMBL1259234 | N-{(2S)-3-(Biphenyl-4-yl)-1-oxo-1-...)Show SMILES CC(C)(C)CC(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(=O)N1CCC[C@H]1C(=O)c1nc2ccccc2[nH]1 |r| Show InChI InChI=1S/C33H36N4O3/c1-33(2,3)21-29(38)34-27(20-22-15-17-24(18-16-22)23-10-5-4-6-11-23)32(40)37-19-9-14-28(37)30(39)31-35-25-12-7-8-13-26(25)36-31/h4-8,10-13,15-18,27-28H,9,14,19-21H2,1-3H3,(H,34,38)(H,35,36)/t27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 399 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
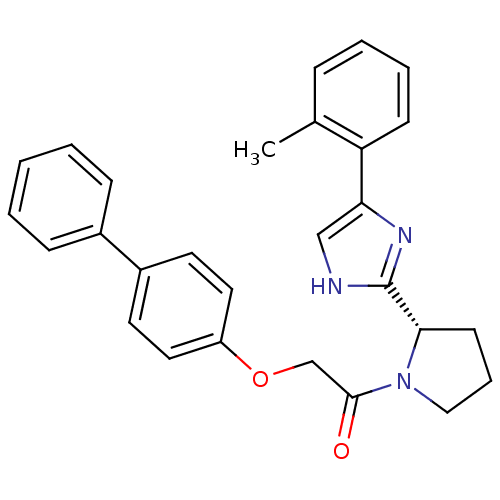
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328581
(2-(Biphenyl-4-yloxy)-1-[(2S)-2-(4-methyl-1H-benzim...)Show SMILES Cc1ccccc1-c1c[nH]c(n1)[C@@H]1CCCN1C(=O)COc1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C28H27N3O2/c1-20-8-5-6-11-24(20)25-18-29-28(30-25)26-12-7-17-31(26)27(32)19-33-23-15-13-22(14-16-23)21-9-3-2-4-10-21/h2-6,8-11,13-16,18,26H,7,12,17,19H2,1H3,(H,29,30)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 432 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
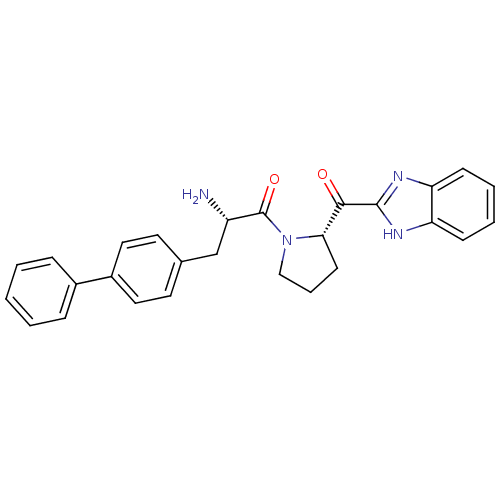
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328552
((2S)-2-Amino-3-(biphenyl-4-yl)-1-[(2S)-2-(4-phenyl...)Show SMILES N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(=O)N1CCC[C@H]1C(=O)c1nc2ccccc2[nH]1 |r| Show InChI InChI=1S/C27H26N4O2/c28-21(17-18-12-14-20(15-13-18)19-7-2-1-3-8-19)27(33)31-16-6-11-24(31)25(32)26-29-22-9-4-5-10-23(22)30-26/h1-5,7-10,12-15,21,24H,6,11,16-17,28H2,(H,29,30)/t21-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 518 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
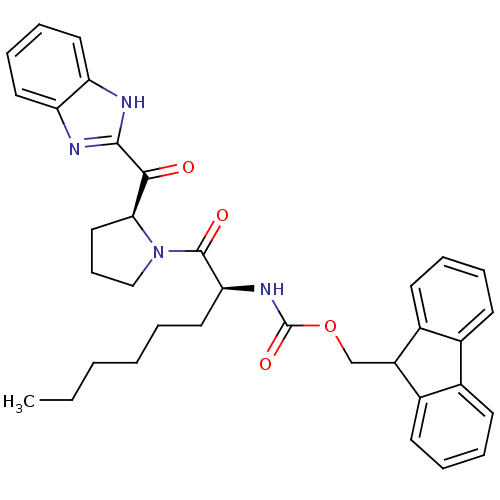
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328548
(9H-Fluoren-9-ylmethyl{(2S)-1-Oxo-1-[(2S)-2-(4-phen...)Show SMILES CCCCCC[C@H](NC(=O)OCC1c2ccccc2-c2ccccc12)C(=O)N1CCC[C@H]1C(=O)c1nc2ccccc2[nH]1 |r| Show InChI InChI=1S/C35H38N4O4/c1-2-3-4-5-19-30(34(41)39-21-12-20-31(39)32(40)33-36-28-17-10-11-18-29(28)37-33)38-35(42)43-22-27-25-15-8-6-13-23(25)24-14-7-9-16-26(24)27/h6-11,13-18,27,30-31H,2-5,12,19-22H2,1H3,(H,36,37)(H,38,42)/t30-,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50328534

(2-Amino-N-{(2S)-3-(biphenyl-4-yl)-1-[(2S)-2-(5,6-d...)Show SMILES CC(C)(N)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C30H31Cl2N5O2/c1-30(2,33)29(39)36-25(15-18-10-12-20(13-11-18)19-7-4-3-5-8-19)28(38)37-14-6-9-26(37)27-34-23-16-21(31)22(32)17-24(23)35-27/h3-5,7-8,10-13,16-17,25-26H,6,9,14-15,33H2,1-2H3,(H,34,35)(H,36,39)/t25-,26-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 789 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP by FRET in presence of 1% mouse serum albumin |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328546

(CHEMBL1256276 | Prop-2-en-1-yl{(5S)-5-{[(9H-Fluore...)Show SMILES C=CCC(=O)OCCCC[C@H](NC(=O)OCC1c2ccccc2-c2ccccc12)C(=O)N1CCC[C@H]1C(=O)c1nc2ccccc2[nH]1 |r| Show InChI InChI=1S/C37H38N4O6/c1-2-12-33(42)46-22-10-9-19-31(36(44)41-21-11-20-32(41)34(43)35-38-29-17-7-8-18-30(29)39-35)40-37(45)47-23-28-26-15-5-3-13-24(26)25-14-4-6-16-27(25)28/h2-8,13-18,28,31-32H,1,9-12,19-23H2,(H,38,39)(H,40,45)/t31-,32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328536

(5,6-Dichloro-2-[(2S)-1-(2-methylalanyl-O-methyl-L-...)Show SMILES COc1ccc(C[C@H](NC(=O)C(C)(C)N)C(=O)N2CC[C@H]2c2nc3cc(Cl)c(Cl)cc3[nH]2)cc1 |r| Show InChI InChI=1S/C24H27Cl2N5O3/c1-24(2,27)23(33)30-19(10-13-4-6-14(34-3)7-5-13)22(32)31-9-8-20(31)21-28-17-11-15(25)16(26)12-18(17)29-21/h4-7,11-12,19-20H,8-10,27H2,1-3H3,(H,28,29)(H,30,33)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 829 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET in presence of 1% mouse serum albumin |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328553

(4'-{(2S)-2-[(tert-Butoxycarbonyl)amino]-3-oxo-3-[(...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccc(cc1)-c1ccc(cc1)C(O)=O)C(=O)N1CCC[C@H]1C(=O)c1nc2ccccc2[nH]1 |r| Show InChI InChI=1S/C33H34N4O6/c1-33(2,3)43-32(42)36-26(19-20-10-12-21(13-11-20)22-14-16-23(17-15-22)31(40)41)30(39)37-18-6-9-27(37)28(38)29-34-24-7-4-5-8-25(24)35-29/h4-5,7-8,10-17,26-27H,6,9,18-19H2,1-3H3,(H,34,35)(H,36,42)(H,40,41)/t26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 849 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
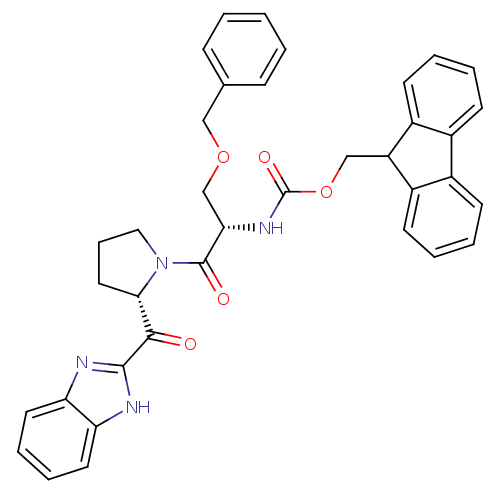
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328544
(9H-Fluoren-9-ylmethyl{(2S)-3-(Benzyloxy)-1-oxo-1-[...)Show SMILES O=C(N[C@@H](COCc1ccccc1)C(=O)N1CCC[C@H]1C(=O)c1nc2ccccc2[nH]1)OCC1c2ccccc2-c2ccccc12 |r| Show InChI InChI=1S/C37H34N4O5/c42-34(35-38-30-17-8-9-18-31(30)39-35)33-19-10-20-41(33)36(43)32(23-45-21-24-11-2-1-3-12-24)40-37(44)46-22-29-27-15-6-4-13-25(27)26-14-5-7-16-28(26)29/h1-9,11-18,29,32-33H,10,19-23H2,(H,38,39)(H,40,44)/t32-,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50328530

(3-(Biphenyl-4-yl)-1-{2-[5-(3-fluorophenyl)-1H-imid...)Show SMILES Fc1cccc(c1)-c1c[nH]c(n1)[C@@H]1CCCN1C(=O)CCc1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C28H26FN3O/c29-24-9-4-8-23(18-24)25-19-30-28(31-25)26-10-5-17-32(26)27(33)16-13-20-11-14-22(15-12-20)21-6-2-1-3-7-21/h1-4,6-9,11-12,14-15,18-19,26H,5,10,13,16-17H2,(H,30,31)/t26-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 871 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328540

(CHEMBL1259130 | N-(1-(2-(benzo[d]oxazole-2-carbony...)Show SMILES CCCCC(NC(C)=O)C(=O)N1CCCC1C(=O)c1nc2ccccc2o1 Show InChI InChI=1S/C20H25N3O4/c1-3-4-8-15(21-13(2)24)20(26)23-12-7-10-16(23)18(25)19-22-14-9-5-6-11-17(14)27-19/h5-6,9,11,15-16H,3-4,7-8,10,12H2,1-2H3,(H,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328531

(3-(Biphenyl-4-yl)-1-{2-[5-(3-fluorophenyl)-1H-imid...)Show SMILES CC(C)(Cc1ccc(cc1)-c1ccccc1)C(=O)N1CCC[C@H]1c1nc(c[nH]1)-c1cccc(F)c1 |r| Show InChI InChI=1S/C30H30FN3O/c1-30(2,19-21-13-15-23(16-14-21)22-8-4-3-5-9-22)29(35)34-17-7-12-27(34)28-32-20-26(33-28)24-10-6-11-25(31)18-24/h3-6,8-11,13-16,18,20,27H,7,12,17,19H2,1-2H3,(H,32,33)/t27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET in presence of 1% mouse serum albumin |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328533

(2-(biphenyl-4-yloxy)-1-((1R,2S,5S)-2-(4-methyl-1H-...)Show SMILES Cc1cccc2[nH]c(nc12)[C@@H]1[C@@H]2C[C@@H]2CN1C(=O)COc1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C27H25N3O2/c1-17-6-5-9-23-25(17)29-27(28-23)26-22-14-20(22)15-30(26)24(31)16-32-21-12-10-19(11-13-21)18-7-3-2-4-8-18/h2-13,20,22,26H,14-16H2,1H3,(H,28,29)/t20-,22-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET in presence of 1% mouse serum albumin |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328530

(3-(Biphenyl-4-yl)-1-{2-[5-(3-fluorophenyl)-1H-imid...)Show SMILES Fc1cccc(c1)-c1c[nH]c(n1)[C@@H]1CCCN1C(=O)CCc1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C28H26FN3O/c29-24-9-4-8-23(18-24)25-19-30-28(31-25)26-10-5-17-32(26)27(33)16-13-20-11-14-22(15-12-20)21-6-2-1-3-7-21/h1-4,6-9,11-12,14-15,18-19,26H,5,10,13,16-17H2,(H,30,31)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET in presence of 1% mouse serum albumin |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50328536

(5,6-Dichloro-2-[(2S)-1-(2-methylalanyl-O-methyl-L-...)Show SMILES COc1ccc(C[C@H](NC(=O)C(C)(C)N)C(=O)N2CC[C@H]2c2nc3cc(Cl)c(Cl)cc3[nH]2)cc1 |r| Show InChI InChI=1S/C24H27Cl2N5O3/c1-24(2,27)23(33)30-19(10-13-4-6-14(34-3)7-5-13)22(32)31-9-8-20(31)21-28-17-11-15(25)16(26)12-18(17)29-21/h4-7,11-12,19-20H,8-10,27H2,1-3H3,(H,28,29)(H,30,33)/t19-,20-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP by FRET in presence of 1% mouse serum albumin |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328555

(3-(3'-Aminobiphenyl-4-yl)-1-[(2S)-2-(4-phenyl-1H-i...)Show SMILES Nc1cccc(c1)-c1ccc(CCC(=O)N2CCC[C@H]2c2nc(c[nH]2)-c2ccccc2)cc1 |r| Show InChI InChI=1S/C28H28N4O/c29-24-9-4-8-23(18-24)21-14-11-20(12-15-21)13-16-27(33)32-17-5-10-26(32)28-30-19-25(31-28)22-6-2-1-3-7-22/h1-4,6-9,11-12,14-15,18-19,26H,5,10,13,16-17,29H2,(H,30,31)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328543
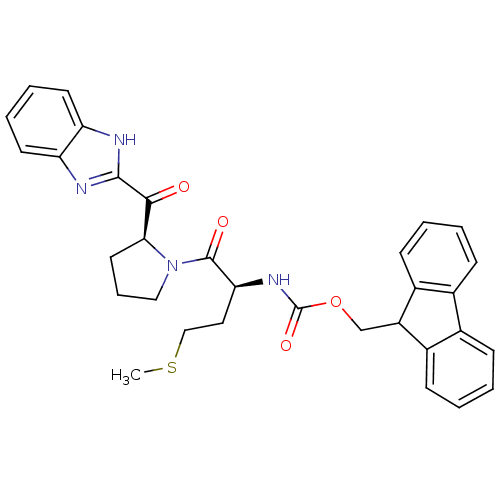
(9H-Fluoren-9-ylmethyl{(2S)-4-(Methylsulfanyl)-1-ox...)Show SMILES CSCC[C@H](NC(=O)OCC1c2ccccc2-c2ccccc12)C(=O)N1CCC[C@H]1C(=O)c1nc2ccccc2[nH]1 |r| Show InChI InChI=1S/C32H32N4O4S/c1-41-18-16-27(31(38)36-17-8-15-28(36)29(37)30-33-25-13-6-7-14-26(25)34-30)35-32(39)40-19-24-22-11-4-2-9-20(22)21-10-3-5-12-23(21)24/h2-7,9-14,24,27-28H,8,15-19H2,1H3,(H,33,34)(H,35,39)/t27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328542

(9H-Fluoren-9-ylmethyl{(2S)-1-Oxo-1-[(2S)-2-(5-phen...)Show SMILES CCCC[C@H](NC(=O)OCC1c2ccccc2-c2ccccc12)C(=O)N1CCC[C@H]1C(=O)c1nc2ccccc2[nH]1 |r| Show InChI InChI=1S/C33H34N4O4/c1-2-3-15-28(32(39)37-19-10-18-29(37)30(38)31-34-26-16-8-9-17-27(26)35-31)36-33(40)41-20-25-23-13-6-4-11-21(23)22-12-5-7-14-24(22)25/h4-9,11-14,16-17,25,28-29H,2-3,10,15,18-20H2,1H3,(H,34,35)(H,36,40)/t28-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328545

(CHEMBL1256274 | tert-Butyl(7S)-7-{[(9H-Fluoren-9-y...)Show SMILES CC(C)(C)OC(=O)CCCCC[C@H](NC(=O)OCC1c2ccccc2-c2ccccc12)C(=O)N1CCC[C@H]1C(=O)c1nc2ccccc2[nH]1 |r| Show InChI InChI=1S/C39H44N4O6/c1-39(2,3)49-34(44)22-6-4-5-20-32(37(46)43-23-13-21-33(43)35(45)36-40-30-18-11-12-19-31(30)41-36)42-38(47)48-24-29-27-16-9-7-14-25(27)26-15-8-10-17-28(26)29/h7-12,14-19,29,32-33H,4-6,13,20-24H2,1-3H3,(H,40,41)(H,42,47)/t32-,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328532

((S)-1-(2-(5-(3-bromophenyl)-1H-imidazol-2-yl)-4,4-...)Show SMILES CC(C)(Cc1ccc(cc1)-c1cccc(Cl)c1Cl)C(=O)N1CC(F)(F)C[C@H]1c1nc(c[nH]1)-c1cccc(Br)c1 |r| Show InChI InChI=1S/C30H26BrCl2F2N3O/c1-29(2,14-18-9-11-19(12-10-18)22-7-4-8-23(32)26(22)33)28(39)38-17-30(34,35)15-25(38)27-36-16-24(37-27)20-5-3-6-21(31)13-20/h3-13,16,25H,14-15,17H2,1-2H3,(H,36,37)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET in presence of 1% mouse serum albumin |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328564

(3-(Biphenyl-4-yl)-1-[(2S)-2-(5-tert-butyl-1H-imida...)Show SMILES CC(C)(C)c1cnc([nH]1)C(=O)[C@@H]1CCCN1C(=O)CCc1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C27H31N3O2/c1-27(2,3)23-18-28-26(29-23)25(32)22-10-7-17-30(22)24(31)16-13-19-11-14-21(15-12-19)20-8-5-4-6-9-20/h4-6,8-9,11-12,14-15,18,22H,7,10,13,16-17H2,1-3H3,(H,28,29)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328570

(3-(Biphenyl-4-yl)-1-{2-[5-(3-chlorophenyl)-1H-imid...)Show SMILES Clc1cccc(c1)-c1c[nH]c(n1)C(=O)[C@@H]1CCCN1C(=O)CCc1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C29H26ClN3O2/c30-24-9-4-8-23(18-24)25-19-31-29(32-25)28(35)26-10-5-17-33(26)27(34)16-13-20-11-14-22(15-12-20)21-6-2-1-3-7-21/h1-4,6-9,11-12,14-15,18-19,26H,5,10,13,16-17H2,(H,31,32)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328567
(3-(Biphenyl-4-yl)-1-{(2S)-2-[5-(3-bromophenyl)-1H-...)Show SMILES Brc1cccc(c1)-c1c[nH]c(n1)C(=O)[C@@H]1CCCN1C(=O)CCc1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C29H26BrN3O2/c30-24-9-4-8-23(18-24)25-19-31-29(32-25)28(35)26-10-5-17-33(26)27(34)16-13-20-11-14-22(15-12-20)21-6-2-1-3-7-21/h1-4,6-9,11-12,14-15,18-19,26H,5,10,13,16-17H2,(H,31,32)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328569
(3-(Biphenyl-4-yl)-1-{(2S)-2-[5-(3-methylphenyl)-1H...)Show SMILES Cc1cccc(c1)-c1c[nH]c(n1)C(=O)[C@@H]1CCCN1C(=O)CCc1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C30H29N3O2/c1-21-7-5-10-25(19-21)26-20-31-30(32-26)29(35)27-11-6-18-33(27)28(34)17-14-22-12-15-24(16-13-22)23-8-3-2-4-9-23/h2-5,7-10,12-13,15-16,19-20,27H,6,11,14,17-18H2,1H3,(H,31,32)/t27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328573

(3-(Biphenyl-4-yl)-1-{2-[5-(furan-2-yl)-1H-imidazol...)Show SMILES O=C(CCc1ccc(cc1)-c1ccccc1)N1CCC[C@H]1C(=O)c1nc(c[nH]1)-c1ccco1 |r| Show InChI InChI=1S/C27H25N3O3/c31-25(15-12-19-10-13-21(14-11-19)20-6-2-1-3-7-20)30-16-4-8-23(30)26(32)27-28-18-22(29-27)24-9-5-17-33-24/h1-3,5-7,9-11,13-14,17-18,23H,4,8,12,15-16H2,(H,28,29)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328579
(3-(Biphenyl-4-yl)-1-[2-(5,6-dichloro-1H-benzimidaz...)Show SMILES Clc1cc2nc([nH]c2cc1Cl)C(=O)[C@@H]1CCCN1C(=O)CCc1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C27H23Cl2N3O2/c28-20-15-22-23(16-21(20)29)31-27(30-22)26(34)24-7-4-14-32(24)25(33)13-10-17-8-11-19(12-9-17)18-5-2-1-3-6-18/h1-3,5-6,8-9,11-12,15-16,24H,4,7,10,13-14H2,(H,30,31)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328565

(3-(Biphenyl-4-yl)-1-{(2S)-2-[5-(prop-1-en-2-yl)-1H...)Show SMILES CC(=C)c1cnc([nH]1)C(=O)[C@@H]1CCCN1C(=O)CCc1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C26H27N3O2/c1-18(2)22-17-27-26(28-22)25(31)23-9-6-16-29(23)24(30)15-12-19-10-13-21(14-11-19)20-7-4-3-5-8-20/h3-5,7-8,10-11,13-14,17,23H,1,6,9,12,15-16H2,2H3,(H,27,28)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328580

(3-(Biphenyl-4-yl)-1-[2-(4,5-dimethyl-1H-benzimidaz...)Show SMILES Cc1ccc2nc([nH]c2c1C)C(=O)[C@@H]1CCCN1C(=O)CCc1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C29H29N3O2/c1-19-10-16-24-27(20(19)2)31-29(30-24)28(34)25-9-6-18-32(25)26(33)17-13-21-11-14-23(15-12-21)22-7-4-3-5-8-22/h3-5,7-8,10-12,14-16,25H,6,9,13,17-18H2,1-2H3,(H,30,31)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.46E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328577
(3-(Biphenyl-4-yl)-1-[2-(5,6-difluoro-1H-benzimidaz...)Show SMILES Fc1cc2nc([nH]c2cc1F)C(=O)[C@@H]1CCCN1C(=O)CCc1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C27H23F2N3O2/c28-20-15-22-23(16-21(20)29)31-27(30-22)26(34)24-7-4-14-32(24)25(33)13-10-17-8-11-19(12-9-17)18-5-2-1-3-6-18/h1-3,5-6,8-9,11-12,15-16,24H,4,7,10,13-14H2,(H,30,31)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.63E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328578

(3-(Biphenyl-4-yl)-1-[2-(6-chloro-5-fluoro-1H-benzi...)Show SMILES Fc1cc2[nH]c(nc2cc1Cl)C(=O)[C@@H]1CCCN1C(=O)CCc1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C27H23ClFN3O2/c28-20-15-22-23(16-21(20)29)31-27(30-22)26(34)24-7-4-14-32(24)25(33)13-10-17-8-11-19(12-9-17)18-5-2-1-3-6-18/h1-3,5-6,8-9,11-12,15-16,24H,4,7,10,13-14H2,(H,30,31)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.84E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328576

(3-(Biphenyl-4-yl)-1-[2-(6-chloro-1H-benzimidazol-2...)Show SMILES Clc1ccc2nc([nH]c2c1)C(=O)[C@@H]1CCCN1C(=O)CCc1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C27H24ClN3O2/c28-21-13-14-22-23(17-21)30-27(29-22)26(33)24-7-4-16-31(24)25(32)15-10-18-8-11-20(12-9-18)19-5-2-1-3-6-19/h1-3,5-6,8-9,11-14,17,24H,4,7,10,15-16H2,(H,29,30)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.21E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328574
(3-(Biphenyl-4-yl)-1-{2-[5-(4-methylthiophen-2-yl)-...)Show SMILES Cc1ccsc1-c1c[nH]c(n1)C(=O)[C@@H]1CCCN1C(=O)CCc1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C28H27N3O2S/c1-19-15-17-34-27(19)23-18-29-28(30-23)26(33)24-8-5-16-31(24)25(32)14-11-20-9-12-22(13-10-20)21-6-3-2-4-7-21/h2-4,6-7,9-10,12-13,15,17-18,24H,5,8,11,14,16H2,1H3,(H,29,30)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.74E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328575

(3-(Biphenyl-4-yl)-1-[2-(6-fluoro-1H-benzimidazol-2...)Show SMILES Fc1ccc2nc([nH]c2c1)C(=O)[C@@H]1CCCN1C(=O)CCc1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C27H24FN3O2/c28-21-13-14-22-23(17-21)30-27(29-22)26(33)24-7-4-16-31(24)25(32)15-10-18-8-11-20(12-9-18)19-5-2-1-3-6-19/h1-3,5-6,8-9,11-14,17,24H,4,7,10,15-16H2,(H,29,30)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328572

(3-(Biphenyl-4-yl)-1-{2-[5-(3-chloro-4-fluorophenyl...)Show SMILES Fc1ccc(cc1Cl)-c1c[nH]c(n1)C(=O)[C@@H]1CCCN1C(=O)CCc1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C29H25ClFN3O2/c30-23-17-22(13-14-24(23)31)25-18-32-29(33-25)28(36)26-7-4-16-34(26)27(35)15-10-19-8-11-21(12-9-19)20-5-2-1-3-6-20/h1-3,5-6,8-9,11-14,17-18,26H,4,7,10,15-16H2,(H,32,33)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328568
(3-(Biphenyl-4-yl)-1-[2-(4-methyl-5-phenyl-1H-imida...)Show SMILES Cc1[nH]c(nc1-c1ccccc1)C(=O)[C@@H]1CCCN1C(=O)CCc1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C30H29N3O2/c1-21-28(25-11-6-3-7-12-25)32-30(31-21)29(35)26-13-8-20-33(26)27(34)19-16-22-14-17-24(18-15-22)23-9-4-2-5-10-23/h2-7,9-12,14-15,17-18,26H,8,13,16,19-20H2,1H3,(H,31,32)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.38E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328563

(3-(Biphenyl-4-yl)-1-{(2S)-2-[5-(2,2,2-trifluoroeth...)Show SMILES FC(F)(F)Cc1cnc([nH]1)C(=O)[C@@H]1CCCN1C(=O)CCc1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C25H24F3N3O2/c26-25(27,28)15-20-16-29-24(30-20)23(33)21-7-4-14-31(21)22(32)13-10-17-8-11-19(12-9-17)18-5-2-1-3-6-18/h1-3,5-6,8-9,11-12,16,21H,4,7,10,13-15H2,(H,29,30)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.76E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328566

(3-(Biphenyl-4-yl)-1-{(2S)-2-[5-(propan-2-yl)-1H-im...)Show SMILES CC(C)c1cnc([nH]1)C(=O)[C@@H]1CCCN1C(=O)CCc1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C26H29N3O2/c1-18(2)22-17-27-26(28-22)25(31)23-9-6-16-29(23)24(30)15-12-19-10-13-21(14-11-19)20-7-4-3-5-8-20/h3-5,7-8,10-11,13-14,17-18,23H,6,9,12,15-16H2,1-2H3,(H,27,28)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328571

(3-(2-{1-[3-(Biphenyl-4-yl)propanoyl]pyrrolidin-2-y...)Show SMILES OC(=O)c1cccc(c1)-c1c[nH]c(n1)C(=O)[C@@H]1CCCN1C(=O)CCc1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C30H27N3O4/c34-27(16-13-20-11-14-22(15-12-20)21-6-2-1-3-7-21)33-17-5-10-26(33)28(35)29-31-19-25(32-29)23-8-4-9-24(18-23)30(36)37/h1-4,6-9,11-12,14-15,18-19,26H,5,10,13,16-17H2,(H,31,32)(H,36,37)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.27E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data