

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50310278 (9-O-[4-(Phenylol-1-yloxy)butyl]berberine bromide |...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institute of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylcholine chloride as substrate preincubated for 15 mins by Ellman's method | Bioorg Med Chem 19: 7228-35 (2011) Article DOI: 10.1016/j.bmc.2011.09.040 BindingDB Entry DOI: 10.7270/Q23F4Q3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359508 (CHEMBL1927128) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institute of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylcholine chloride as substrate preincubated for 15 mins by Ellman's method | Bioorg Med Chem 19: 7228-35 (2011) Article DOI: 10.1016/j.bmc.2011.09.040 BindingDB Entry DOI: 10.7270/Q23F4Q3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359513 (CHEMBL1927133) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 304 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institute of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylcholine chloride as substrate preincubated for 15 mins by Ellman's method | Bioorg Med Chem 19: 7228-35 (2011) Article DOI: 10.1016/j.bmc.2011.09.040 BindingDB Entry DOI: 10.7270/Q23F4Q3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50203126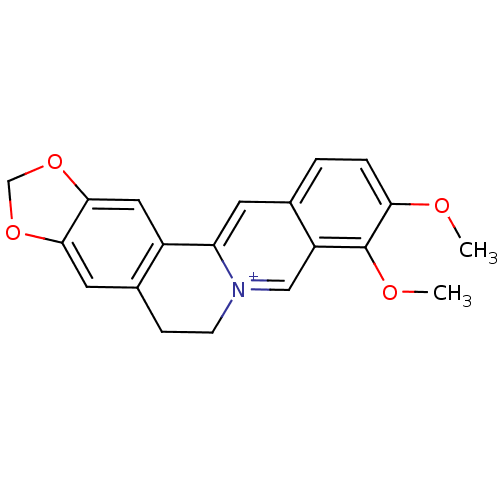 (3,4-dimethoxy-6,7-dihydro-[1,3]dioxolo[4,5-g]pyrid...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 374 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institute of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylcholine chloride as substrate preincubated for 15 mins by Ellman's method | Bioorg Med Chem 19: 7228-35 (2011) Article DOI: 10.1016/j.bmc.2011.09.040 BindingDB Entry DOI: 10.7270/Q23F4Q3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359509 (CHEMBL1927129) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 422 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institute of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylcholine chloride as substrate preincubated for 15 mins by Ellman's method | Bioorg Med Chem 19: 7228-35 (2011) Article DOI: 10.1016/j.bmc.2011.09.040 BindingDB Entry DOI: 10.7270/Q23F4Q3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359512 (CHEMBL1927132) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institute of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylcholine chloride as substrate preincubated for 15 mins by Ellman's method | Bioorg Med Chem 19: 7228-35 (2011) Article DOI: 10.1016/j.bmc.2011.09.040 BindingDB Entry DOI: 10.7270/Q23F4Q3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359507 (CHEMBL1927127) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institute of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylcholine chloride as substrate preincubated for 15 mins by Ellman's method | Bioorg Med Chem 19: 7228-35 (2011) Article DOI: 10.1016/j.bmc.2011.09.040 BindingDB Entry DOI: 10.7270/Q23F4Q3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359511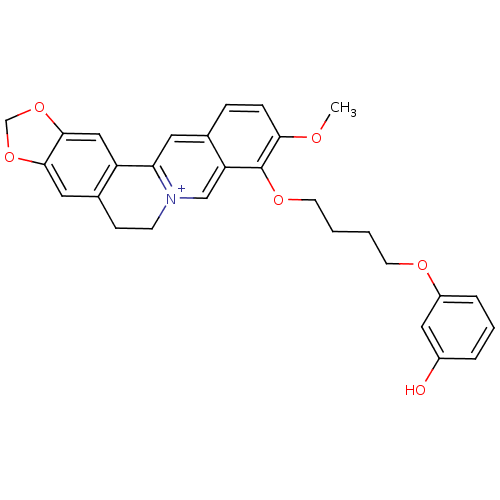 (CHEMBL1927131) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 547 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institute of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylcholine chloride as substrate preincubated for 15 mins by Ellman's method | Bioorg Med Chem 19: 7228-35 (2011) Article DOI: 10.1016/j.bmc.2011.09.040 BindingDB Entry DOI: 10.7270/Q23F4Q3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 623 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institute of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylcholine chloride as substrate preincubated for 15 mins by Ellman's method | Bioorg Med Chem 19: 7228-35 (2011) Article DOI: 10.1016/j.bmc.2011.09.040 BindingDB Entry DOI: 10.7270/Q23F4Q3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359510 (CHEMBL1927130) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 628 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institute of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylcholine chloride as substrate preincubated for 15 mins by Ellman's method | Bioorg Med Chem 19: 7228-35 (2011) Article DOI: 10.1016/j.bmc.2011.09.040 BindingDB Entry DOI: 10.7270/Q23F4Q3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359510 (CHEMBL1927130) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 898 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institute of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using acetylcholine as substrate preincubated for 15 mins by Ellman's method | Bioorg Med Chem 19: 7228-35 (2011) Article DOI: 10.1016/j.bmc.2011.09.040 BindingDB Entry DOI: 10.7270/Q23F4Q3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359513 (CHEMBL1927133) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institute of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using acetylcholine as substrate preincubated for 15 mins by Ellman's method | Bioorg Med Chem 19: 7228-35 (2011) Article DOI: 10.1016/j.bmc.2011.09.040 BindingDB Entry DOI: 10.7270/Q23F4Q3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359513 (CHEMBL1927133) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institute of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 15 mins by Ellman's method | Bioorg Med Chem 19: 7228-35 (2011) Article DOI: 10.1016/j.bmc.2011.09.040 BindingDB Entry DOI: 10.7270/Q23F4Q3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359514 (CHEMBL1927134) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institute of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylcholine chloride as substrate preincubated for 15 mins by Ellman's method | Bioorg Med Chem 19: 7228-35 (2011) Article DOI: 10.1016/j.bmc.2011.09.040 BindingDB Entry DOI: 10.7270/Q23F4Q3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359510 (CHEMBL1927130) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institute of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 15 mins by Ellman's method | Bioorg Med Chem 19: 7228-35 (2011) Article DOI: 10.1016/j.bmc.2011.09.040 BindingDB Entry DOI: 10.7270/Q23F4Q3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359507 (CHEMBL1927127) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institute of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using acetylcholine as substrate preincubated for 15 mins by Ellman's method | Bioorg Med Chem 19: 7228-35 (2011) Article DOI: 10.1016/j.bmc.2011.09.040 BindingDB Entry DOI: 10.7270/Q23F4Q3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359515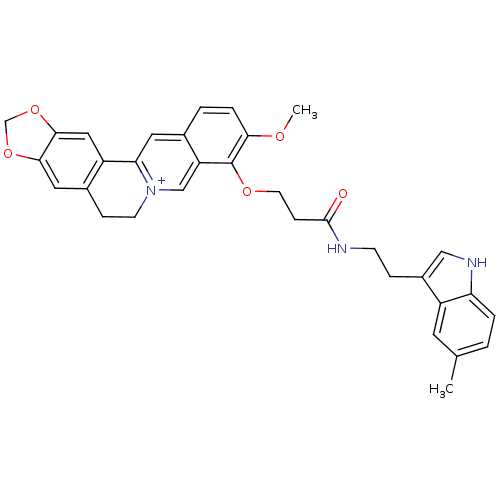 (CHEMBL1927135) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institute of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylcholine chloride as substrate preincubated for 15 mins by Ellman's method | Bioorg Med Chem 19: 7228-35 (2011) Article DOI: 10.1016/j.bmc.2011.09.040 BindingDB Entry DOI: 10.7270/Q23F4Q3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359516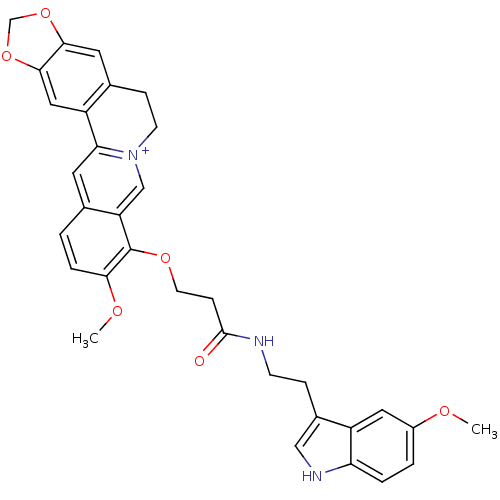 (CHEMBL1927136) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institute of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylcholine chloride as substrate preincubated for 15 mins by Ellman's method | Bioorg Med Chem 19: 7228-35 (2011) Article DOI: 10.1016/j.bmc.2011.09.040 BindingDB Entry DOI: 10.7270/Q23F4Q3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359514 (CHEMBL1927134) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institute of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using acetylcholine as substrate preincubated for 15 mins by Ellman's method | Bioorg Med Chem 19: 7228-35 (2011) Article DOI: 10.1016/j.bmc.2011.09.040 BindingDB Entry DOI: 10.7270/Q23F4Q3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359514 (CHEMBL1927134) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institute of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 15 mins by Ellman's method | Bioorg Med Chem 19: 7228-35 (2011) Article DOI: 10.1016/j.bmc.2011.09.040 BindingDB Entry DOI: 10.7270/Q23F4Q3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359512 (CHEMBL1927132) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institute of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using acetylcholine as substrate preincubated for 15 mins by Ellman's method | Bioorg Med Chem 19: 7228-35 (2011) Article DOI: 10.1016/j.bmc.2011.09.040 BindingDB Entry DOI: 10.7270/Q23F4Q3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359508 (CHEMBL1927128) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institute of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using acetylcholine as substrate preincubated for 15 mins by Ellman's method | Bioorg Med Chem 19: 7228-35 (2011) Article DOI: 10.1016/j.bmc.2011.09.040 BindingDB Entry DOI: 10.7270/Q23F4Q3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359507 (CHEMBL1927127) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institute of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 15 mins by Ellman's method | Bioorg Med Chem 19: 7228-35 (2011) Article DOI: 10.1016/j.bmc.2011.09.040 BindingDB Entry DOI: 10.7270/Q23F4Q3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359515 (CHEMBL1927135) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institute of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using acetylcholine as substrate preincubated for 15 mins by Ellman's method | Bioorg Med Chem 19: 7228-35 (2011) Article DOI: 10.1016/j.bmc.2011.09.040 BindingDB Entry DOI: 10.7270/Q23F4Q3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359511 (CHEMBL1927131) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institute of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using acetylcholine as substrate preincubated for 15 mins by Ellman's method | Bioorg Med Chem 19: 7228-35 (2011) Article DOI: 10.1016/j.bmc.2011.09.040 BindingDB Entry DOI: 10.7270/Q23F4Q3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359515 (CHEMBL1927135) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institute of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 15 mins by Ellman's method | Bioorg Med Chem 19: 7228-35 (2011) Article DOI: 10.1016/j.bmc.2011.09.040 BindingDB Entry DOI: 10.7270/Q23F4Q3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359512 (CHEMBL1927132) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institute of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 15 mins by Ellman's method | Bioorg Med Chem 19: 7228-35 (2011) Article DOI: 10.1016/j.bmc.2011.09.040 BindingDB Entry DOI: 10.7270/Q23F4Q3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359508 (CHEMBL1927128) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institute of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 15 mins by Ellman's method | Bioorg Med Chem 19: 7228-35 (2011) Article DOI: 10.1016/j.bmc.2011.09.040 BindingDB Entry DOI: 10.7270/Q23F4Q3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359517 (CHEMBL1927137) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institute of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using acetylcholine as substrate preincubated for 15 mins by Ellman's method | Bioorg Med Chem 19: 7228-35 (2011) Article DOI: 10.1016/j.bmc.2011.09.040 BindingDB Entry DOI: 10.7270/Q23F4Q3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359509 (CHEMBL1927129) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institute of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using acetylcholine as substrate preincubated for 15 mins by Ellman's method | Bioorg Med Chem 19: 7228-35 (2011) Article DOI: 10.1016/j.bmc.2011.09.040 BindingDB Entry DOI: 10.7270/Q23F4Q3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359511 (CHEMBL1927131) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institute of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 15 mins by Ellman's method | Bioorg Med Chem 19: 7228-35 (2011) Article DOI: 10.1016/j.bmc.2011.09.040 BindingDB Entry DOI: 10.7270/Q23F4Q3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359517 (CHEMBL1927137) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institute of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 15 mins by Ellman's method | Bioorg Med Chem 19: 7228-35 (2011) Article DOI: 10.1016/j.bmc.2011.09.040 BindingDB Entry DOI: 10.7270/Q23F4Q3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359516 (CHEMBL1927136) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institute of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using acetylcholine as substrate preincubated for 15 mins by Ellman's method | Bioorg Med Chem 19: 7228-35 (2011) Article DOI: 10.1016/j.bmc.2011.09.040 BindingDB Entry DOI: 10.7270/Q23F4Q3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359509 (CHEMBL1927129) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institute of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 15 mins by Ellman's method | Bioorg Med Chem 19: 7228-35 (2011) Article DOI: 10.1016/j.bmc.2011.09.040 BindingDB Entry DOI: 10.7270/Q23F4Q3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359516 (CHEMBL1927136) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institute of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 15 mins by Ellman's method | Bioorg Med Chem 19: 7228-35 (2011) Article DOI: 10.1016/j.bmc.2011.09.040 BindingDB Entry DOI: 10.7270/Q23F4Q3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359517 (CHEMBL1927137) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institute of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylcholine chloride as substrate preincubated for 15 mins by Ellman's method | Bioorg Med Chem 19: 7228-35 (2011) Article DOI: 10.1016/j.bmc.2011.09.040 BindingDB Entry DOI: 10.7270/Q23F4Q3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50310278 (9-O-[4-(Phenylol-1-yloxy)butyl]berberine bromide |...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institute of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 15 mins by Ellman's method | Bioorg Med Chem 19: 7228-35 (2011) Article DOI: 10.1016/j.bmc.2011.09.040 BindingDB Entry DOI: 10.7270/Q23F4Q3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institute of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 15 mins by Ellman's method | Bioorg Med Chem 19: 7228-35 (2011) Article DOI: 10.1016/j.bmc.2011.09.040 BindingDB Entry DOI: 10.7270/Q23F4Q3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50203126 (3,4-dimethoxy-6,7-dihydro-[1,3]dioxolo[4,5-g]pyrid...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institute of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 15 mins by Ellman's method | Bioorg Med Chem 19: 7228-35 (2011) Article DOI: 10.1016/j.bmc.2011.09.040 BindingDB Entry DOI: 10.7270/Q23F4Q3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50214744 ((2E)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoic aci...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institute of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylcholine chloride as substrate preincubated for 15 mins by Ellman's method | Bioorg Med Chem 19: 7228-35 (2011) Article DOI: 10.1016/j.bmc.2011.09.040 BindingDB Entry DOI: 10.7270/Q23F4Q3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50067040 (((E,E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-hept...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institute of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylcholine chloride as substrate preincubated for 15 mins by Ellman's method | Bioorg Med Chem 19: 7228-35 (2011) Article DOI: 10.1016/j.bmc.2011.09.040 BindingDB Entry DOI: 10.7270/Q23F4Q3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||