

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM213823 (EPL-BS1246 (UDO)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Vanderbilt University | Assay Description Inhibition of hepatic cytochromes P450 was assessed in human liver microsomes using a substrate-specific approach of monitoring metabolites formed by... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM213822 (EPL-BS0967 (UDD)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Vanderbilt University | Assay Description Inhibition of hepatic cytochromes P450 was assessed in human liver microsomes using a substrate-specific approach of monitoring metabolites formed by... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM213823 (EPL-BS1246 (UDO)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Vanderbilt University | Assay Description Inhibition of hepatic cytochromes P450 was assessed in human liver microsomes using a substrate-specific approach of monitoring metabolites formed by... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM213822 (EPL-BS0967 (UDD)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Vanderbilt University | Assay Description Inhibition of hepatic cytochromes P450 was assessed in human liver microsomes using a substrate-specific approach of monitoring metabolites formed by... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM213823 (EPL-BS1246 (UDO)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vanderbilt University | Assay Description Inhibition of hepatic cytochromes P450 was assessed in human liver microsomes using a substrate-specific approach of monitoring metabolites formed by... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM213822 (EPL-BS0967 (UDD)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vanderbilt University | Assay Description Inhibition of hepatic cytochromes P450 was assessed in human liver microsomes using a substrate-specific approach of monitoring metabolites formed by... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM213823 (EPL-BS1246 (UDO)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vanderbilt University | Assay Description Inhibition of hepatic cytochromes P450 was assessed in human liver microsomes using a substrate-specific approach of monitoring metabolites formed by... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM213822 (EPL-BS0967 (UDD)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vanderbilt University | Assay Description Inhibition of hepatic cytochromes P450 was assessed in human liver microsomes using a substrate-specific approach of monitoring metabolites formed by... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM213822 (EPL-BS0967 (UDD)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vanderbilt University | Assay Description Inhibition of hepatic cytochromes P450 was assessed in human liver microsomes using a substrate-specific approach of monitoring metabolites formed by... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM213823 (EPL-BS1246 (UDO)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vanderbilt University | Assay Description Inhibition of hepatic cytochromes P450 was assessed in human liver microsomes using a substrate-specific approach of monitoring metabolites formed by... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol 14-alpha demethylase (Trypanosoma cruzi) | BDBM213823 (EPL-BS1246 (UDO)) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | n/a | 19 | n/a | n/a | n/a | 7.4 | 37 |
Vanderbilt University | Assay Description Briefly, the reaction mixture contained 1 uM CYP51, 2 uM cytochrome P450 reductase, 100 uM dilauroyl-alpha-phosphatidylcholine, 0.4 mg/ml isoctrate d... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sterol 14-alpha demethylase (Trypanosoma cruzi) | BDBM213825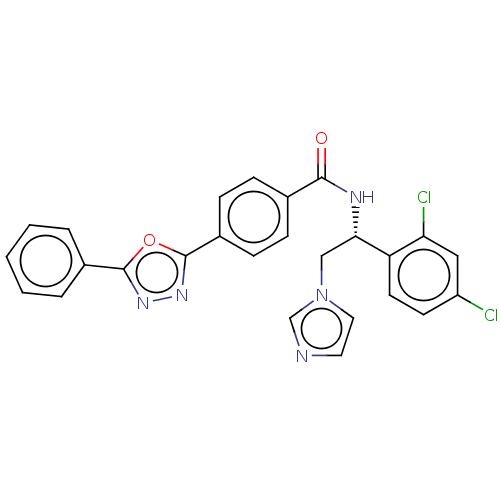 (VNI) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | n/a | 15 | n/a | n/a | n/a | 7.4 | 37 |
Vanderbilt University | Assay Description Briefly, the reaction mixture contained 1 uM CYP51, 2 uM cytochrome P450 reductase, 100 uM dilauroyl-alpha-phosphatidylcholine, 0.4 mg/ml isoctrate d... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sterol 14-alpha demethylase (Trypanosoma cruzi) | BDBM213822 (EPL-BS0967 (UDD)) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | n/a | 32 | n/a | n/a | n/a | 7.4 | 37 |
Vanderbilt University | Assay Description Briefly, the reaction mixture contained 1 uM CYP51, 2 uM cytochrome P450 reductase, 100 uM dilauroyl-alpha-phosphatidylcholine, 0.4 mg/ml isoctrate d... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Putative lanosterol 14-alpha-demethylase (Leishmania infantum) | BDBM213823 (EPL-BS1246 (UDO)) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | 156 | n/a | n/a | n/a | 7.4 | 37 |
Vanderbilt University | Assay Description Briefly, the reaction mixture contained 1 uM CYP51, 2 uM cytochrome P450 reductase, 100 uM dilauroyl-alpha-phosphatidylcholine, 0.4 mg/ml isoctrate d... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol 14-alpha demethylase (Homo sapiens (Human)) | BDBM213822 (EPL-BS0967 (UDD)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | 26 | n/a | n/a | n/a | 7.4 | 37 |
Vanderbilt University | Assay Description Briefly, the reaction mixture contained 1 uM CYP51, 2 uM cytochrome P450 reductase, 100 uM dilauroyl-alpha-phosphatidylcholine, 0.4 mg/ml isoctrate d... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Putative lanosterol 14-alpha-demethylase (Leishmania infantum) | BDBM213824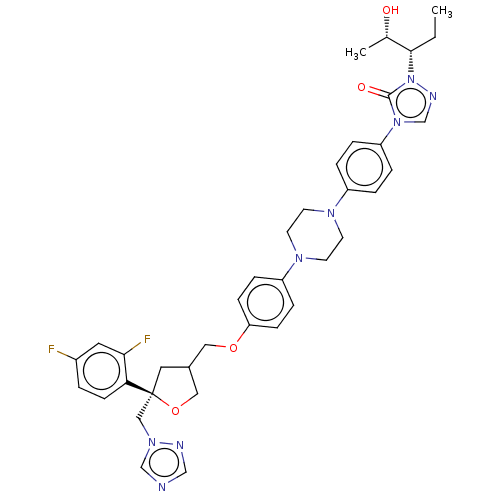 (Posaconazole) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 18 | n/a | n/a | n/a | 7.4 | 37 |
Vanderbilt University | Assay Description Briefly, the reaction mixture contained 1 uM CYP51, 2 uM cytochrome P450 reductase, 100 uM dilauroyl-alpha-phosphatidylcholine, 0.4 mg/ml isoctrate d... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol 14-alpha demethylase (Trypanosoma cruzi) | BDBM213824 (Posaconazole) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 18 | n/a | n/a | n/a | 7.4 | 37 |
Vanderbilt University | Assay Description Briefly, the reaction mixture contained 1 uM CYP51, 2 uM cytochrome P450 reductase, 100 uM dilauroyl-alpha-phosphatidylcholine, 0.4 mg/ml isoctrate d... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol 14-alpha demethylase (Homo sapiens (Human)) | BDBM213823 (EPL-BS1246 (UDO)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | 69 | n/a | n/a | n/a | 7.4 | 37 |
Vanderbilt University | Assay Description Briefly, the reaction mixture contained 1 uM CYP51, 2 uM cytochrome P450 reductase, 100 uM dilauroyl-alpha-phosphatidylcholine, 0.4 mg/ml isoctrate d... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Putative lanosterol 14-alpha-demethylase (Leishmania infantum) | BDBM213822 (EPL-BS0967 (UDD)) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | 419 | n/a | n/a | n/a | 7.4 | 37 |
Vanderbilt University | Assay Description Briefly, the reaction mixture contained 1 uM CYP51, 2 uM cytochrome P450 reductase, 100 uM dilauroyl-alpha-phosphatidylcholine, 0.4 mg/ml isoctrate d... | J Biol Chem 288: 31602-15 (2013) Article DOI: 10.1074/jbc.M113.497990 BindingDB Entry DOI: 10.7270/Q24F1PKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||