 Found 96 hits of Enzyme Inhibition Constant Data
Found 96 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50435350
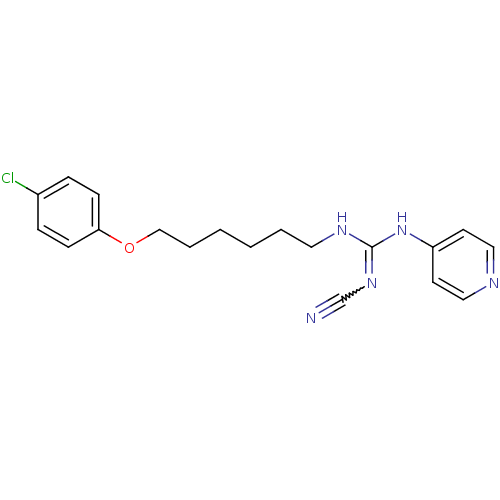
(CHEMBL17289)Show SMILES Clc1ccc(OCCCCCCNC(Nc2ccncc2)=NC#N)cc1 |w:21.22| Show InChI InChI=1S/C19H22ClN5O/c20-16-5-7-18(8-6-16)26-14-4-2-1-3-11-23-19(24-15-21)25-17-9-12-22-13-10-17/h5-10,12-13H,1-4,11,14H2,(H2,22,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged human full-length NAMPT expressed in Escherichia coli Rosetta DE3 using nicotinamide as substrate preincubated fo... |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50448625
(CHEMBL3127508)Show SMILES CC(C)(O)Cn1cc(cn1)S(=O)(=O)c1ccc(NC(=O)[C@@H]2C[C@H]2c2cccnc2)cc1 |r| Show InChI InChI=1S/C22H24N4O4S/c1-22(2,28)14-26-13-18(12-24-26)31(29,30)17-7-5-16(6-8-17)25-21(27)20-10-19(20)15-4-3-9-23-11-15/h3-9,11-13,19-20,28H,10,14H2,1-2H3,(H,25,27)/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged human full-length NAMPT expressed in Escherichia coli Rosetta DE3 using nicotinamide as substrate preincubated fo... |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50435774

(CHEMBL2393183 | US11279687, Compound 204)Show SMILES Nc1ccc(CNC(=O)Nc2ccc(cc2)S(=O)(=O)c2ccccc2)cn1 Show InChI InChI=1S/C19H18N4O3S/c20-18-11-6-14(12-21-18)13-22-19(24)23-15-7-9-17(10-8-15)27(25,26)16-4-2-1-3-5-16/h1-12H,13H2,(H2,20,21)(H2,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged human full-length NAMPT expressed in Escherichia coli Rosetta DE3 using nicotinamide as substrate preincubated fo... |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM81395
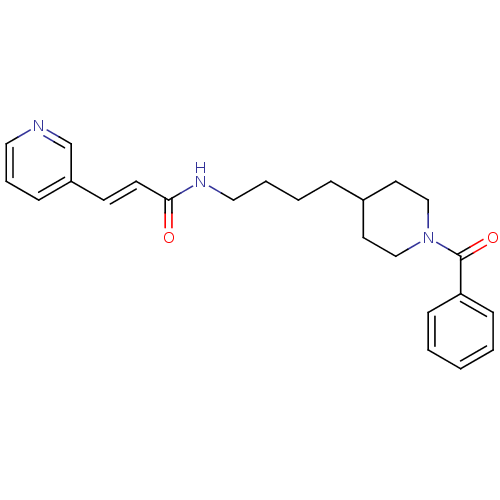
(APO-866)Show SMILES O=C(NCCCCC1CCN(CC1)C(=O)c1ccccc1)\C=C\c1cccnc1 Show InChI InChI=1S/C24H29N3O2/c28-23(12-11-21-8-6-15-25-19-21)26-16-5-4-7-20-13-17-27(18-14-20)24(29)22-9-2-1-3-10-22/h1-3,6,8-12,15,19-20H,4-5,7,13-14,16-18H2,(H,26,28)/b12-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| MMDB
PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged human full-length NAMPT expressed in Escherichia coli Rosetta DE3 using nicotinamide as substrate preincubated fo... |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50448623
(CHEMBL3127493)Show SMILES CC(C)n1cc(c(C)n1)S(=O)(=O)c1ccc(NC(=O)[C@@H]2C[C@H]2c2cccnc2)cc1 |r| Show InChI InChI=1S/C22H24N4O3S/c1-14(2)26-13-21(15(3)25-26)30(28,29)18-8-6-17(7-9-18)24-22(27)20-11-19(20)16-5-4-10-23-12-16/h4-10,12-14,19-20H,11H2,1-3H3,(H,24,27)/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged human full-length NAMPT expressed in Escherichia coli Rosetta DE3 using nicotinamide as substrate preincubated fo... |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50448621
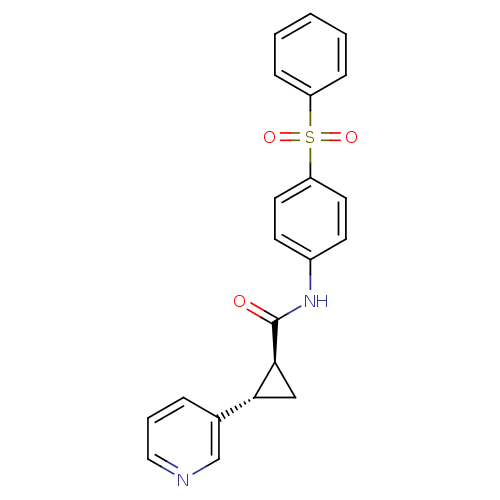
(CHEMBL3127521)Show SMILES O=C(Nc1ccc(cc1)S(=O)(=O)c1ccccc1)[C@H]1C[C@@H]1c1cccnc1 |r| Show InChI InChI=1S/C21H18N2O3S/c24-21(20-13-19(20)15-5-4-12-22-14-15)23-16-8-10-18(11-9-16)27(25,26)17-6-2-1-3-7-17/h1-12,14,19-20H,13H2,(H,23,24)/t19-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged human full-length NAMPT expressed in Escherichia coli Rosetta DE3 using nicotinamide as substrate preincubated fo... |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50448617
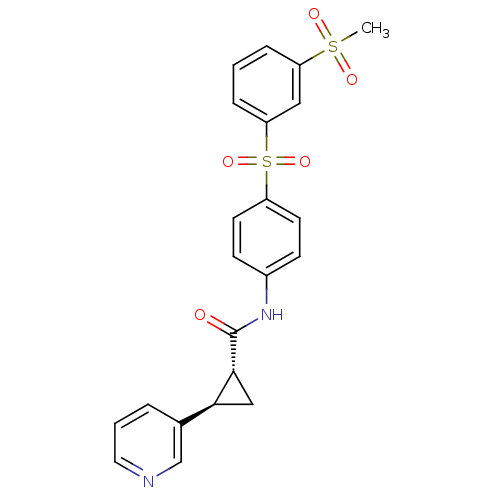
(CHEMBL3127500)Show SMILES CS(=O)(=O)c1cccc(c1)S(=O)(=O)c1ccc(NC(=O)[C@@H]2C[C@H]2c2cccnc2)cc1 |r| Show InChI InChI=1S/C22H20N2O5S2/c1-30(26,27)18-5-2-6-19(12-18)31(28,29)17-9-7-16(8-10-17)24-22(25)21-13-20(21)15-4-3-11-23-14-15/h2-12,14,20-21H,13H2,1H3,(H,24,25)/t20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged human full-length NAMPT expressed in Escherichia coli Rosetta DE3 using nicotinamide as substrate preincubated fo... |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50448632

(CHEMBL3127497)Show SMILES O=C(NCCCCC1CCN(CC1)C(=O)c1ccccc1)[C@@H]1C[C@H]1c1cccnc1 |r| Show InChI InChI=1S/C25H31N3O2/c29-24(23-17-22(23)21-10-6-13-26-18-21)27-14-5-4-7-19-11-15-28(16-12-19)25(30)20-8-2-1-3-9-20/h1-3,6,8-10,13,18-19,22-23H,4-5,7,11-12,14-17H2,(H,27,29)/t22-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged human full-length NAMPT expressed in Escherichia coli Rosetta DE3 using nicotinamide as substrate preincubated fo... |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50448612
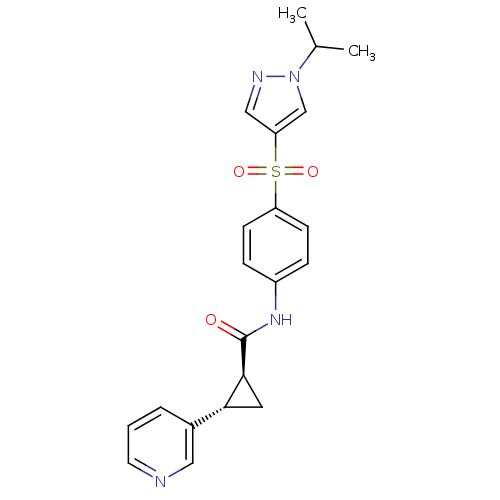
(CHEMBL3127510)Show SMILES CC(C)n1cc(cn1)S(=O)(=O)c1ccc(NC(=O)[C@H]2C[C@@H]2c2cccnc2)cc1 |r| Show InChI InChI=1S/C21H22N4O3S/c1-14(2)25-13-18(12-23-25)29(27,28)17-7-5-16(6-8-17)24-21(26)20-10-19(20)15-4-3-9-22-11-15/h3-9,11-14,19-20H,10H2,1-2H3,(H,24,26)/t19-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged human full-length NAMPT expressed in Escherichia coli Rosetta DE3 using nicotinamide as substrate preincubated fo... |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50448614
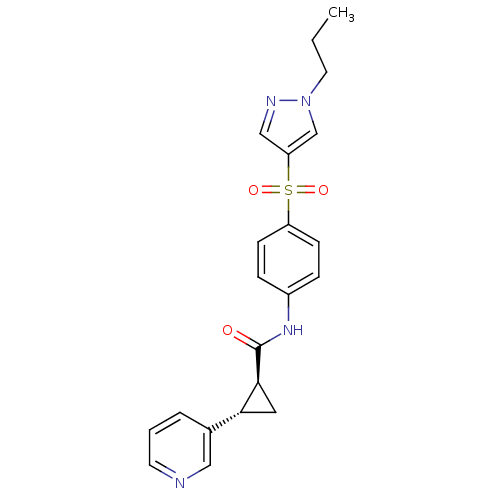
(CHEMBL3127507)Show SMILES CCCn1cc(cn1)S(=O)(=O)c1ccc(NC(=O)[C@H]2C[C@@H]2c2cccnc2)cc1 |r| Show InChI InChI=1S/C21H22N4O3S/c1-2-10-25-14-18(13-23-25)29(27,28)17-7-5-16(6-8-17)24-21(26)20-11-19(20)15-4-3-9-22-12-15/h3-9,12-14,19-20H,2,10-11H2,1H3,(H,24,26)/t19-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged human full-length NAMPT expressed in Escherichia coli Rosetta DE3 using nicotinamide as substrate preincubated fo... |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50448611

(CHEMBL3127494)Show SMILES O=C(Nc1ccc(cc1)S(=O)(=O)c1cnn(c1)C1CCOCC1)[C@@H]1C[C@H]1c1cccnc1 |r| Show InChI InChI=1S/C23H24N4O4S/c28-23(22-12-21(22)16-2-1-9-24-13-16)26-17-3-5-19(6-4-17)32(29,30)20-14-25-27(15-20)18-7-10-31-11-8-18/h1-6,9,13-15,18,21-22H,7-8,10-12H2,(H,26,28)/t21-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged human full-length NAMPT expressed in Escherichia coli Rosetta DE3 using nicotinamide as substrate preincubated fo... |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50448615

(CHEMBL3127506)Show SMILES CCCn1cc(cn1)S(=O)(=O)c1ccc(NC(=O)[C@@H]2C[C@H]2c2cccnc2)cc1 |r| Show InChI InChI=1S/C21H22N4O3S/c1-2-10-25-14-18(13-23-25)29(27,28)17-7-5-16(6-8-17)24-21(26)20-11-19(20)15-4-3-9-22-12-15/h3-9,12-14,19-20H,2,10-11H2,1H3,(H,24,26)/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged human full-length NAMPT expressed in Escherichia coli Rosetta DE3 using nicotinamide as substrate preincubated fo... |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50448631

(CHEMBL3127498)Show SMILES Clc1ccc(OCCCCCCNC(=O)[C@@H]2C[C@H]2c2cccnc2)cc1 |r| Show InChI InChI=1S/C21H25ClN2O2/c22-17-7-9-18(10-8-17)26-13-4-2-1-3-12-24-21(25)20-14-19(20)16-6-5-11-23-15-16/h5-11,15,19-20H,1-4,12-14H2,(H,24,25)/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged human full-length NAMPT expressed in Escherichia coli Rosetta DE3 using nicotinamide as substrate preincubated fo... |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50448619

(CHEMBL3127525)Show SMILES O=C(Nc1ccc(cc1)S(=O)(=O)N1CC2CCC(C1)O2)[C@@H]1C[C@H]1c1cccnc1 |r| Show InChI InChI=1S/C21H23N3O4S/c25-21(20-10-19(20)14-2-1-9-22-11-14)23-15-3-7-18(8-4-15)29(26,27)24-12-16-5-6-17(13-24)28-16/h1-4,7-9,11,16-17,19-20H,5-6,10,12-13H2,(H,23,25)/t16?,17?,19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged human full-length NAMPT expressed in Escherichia coli Rosetta DE3 using nicotinamide as substrate preincubated fo... |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50448627
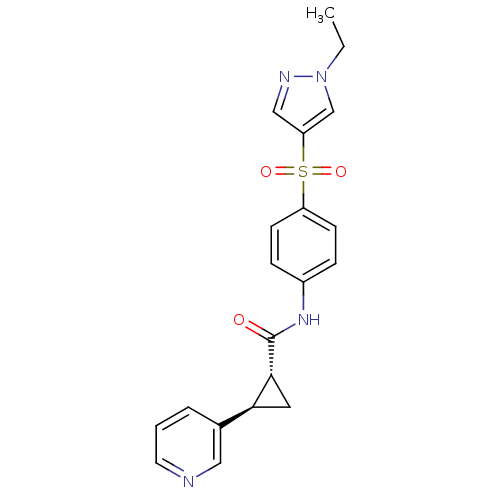
(CHEMBL3127504)Show SMILES CCn1cc(cn1)S(=O)(=O)c1ccc(NC(=O)[C@@H]2C[C@H]2c2cccnc2)cc1 |r| Show InChI InChI=1S/C20H20N4O3S/c1-2-24-13-17(12-22-24)28(26,27)16-7-5-15(6-8-16)23-20(25)19-10-18(19)14-4-3-9-21-11-14/h3-9,11-13,18-19H,2,10H2,1H3,(H,23,25)/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged human full-length NAMPT expressed in Escherichia coli Rosetta DE3 using nicotinamide as substrate preincubated fo... |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50448616
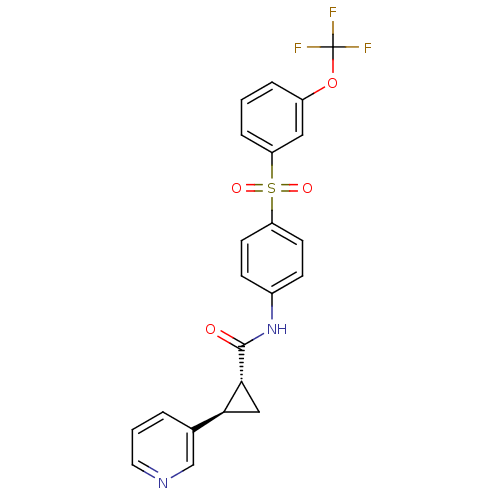
(CHEMBL3127501)Show SMILES FC(F)(F)Oc1cccc(c1)S(=O)(=O)c1ccc(NC(=O)[C@@H]2C[C@H]2c2cccnc2)cc1 |r| Show InChI InChI=1S/C22H17F3N2O4S/c23-22(24,25)31-16-4-1-5-18(11-16)32(29,30)17-8-6-15(7-9-17)27-21(28)20-12-19(20)14-3-2-10-26-13-14/h1-11,13,19-20H,12H2,(H,27,28)/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged human full-length NAMPT expressed in Escherichia coli Rosetta DE3 using nicotinamide as substrate preincubated fo... |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50448629

(CHEMBL3127502)Show SMILES FC(F)(F)c1cccc(c1)S(=O)(=O)c1ccc(NC(=O)[C@@H]2C[C@H]2c2cccnc2)cc1 |r| Show InChI InChI=1S/C22H17F3N2O3S/c23-22(24,25)15-4-1-5-18(11-15)31(29,30)17-8-6-16(7-9-17)27-21(28)20-12-19(20)14-3-2-10-26-13-14/h1-11,13,19-20H,12H2,(H,27,28)/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged human full-length NAMPT expressed in Escherichia coli Rosetta DE3 using nicotinamide as substrate preincubated fo... |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50448626
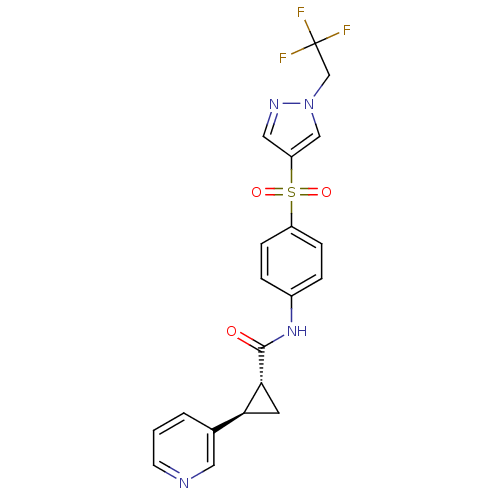
(CHEMBL3127505)Show SMILES FC(F)(F)Cn1cc(cn1)S(=O)(=O)c1ccc(NC(=O)[C@@H]2C[C@H]2c2cccnc2)cc1 |r| Show InChI InChI=1S/C20H17F3N4O3S/c21-20(22,23)12-27-11-16(10-25-27)31(29,30)15-5-3-14(4-6-15)26-19(28)18-8-17(18)13-2-1-7-24-9-13/h1-7,9-11,17-18H,8,12H2,(H,26,28)/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged human full-length NAMPT expressed in Escherichia coli Rosetta DE3 using nicotinamide as substrate preincubated fo... |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50448624
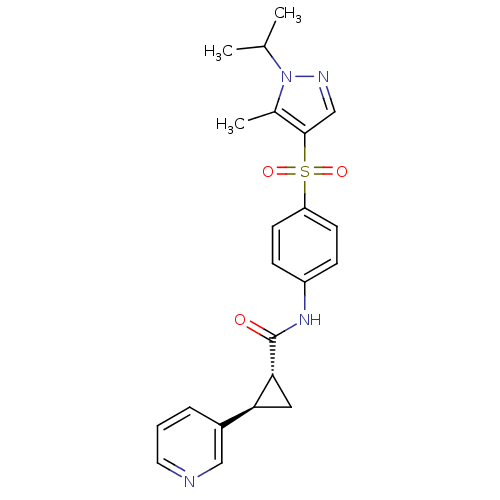
(CHEMBL3127511)Show SMILES CC(C)n1ncc(c1C)S(=O)(=O)c1ccc(NC(=O)[C@@H]2C[C@H]2c2cccnc2)cc1 |r| Show InChI InChI=1S/C22H24N4O3S/c1-14(2)26-15(3)21(13-24-26)30(28,29)18-8-6-17(7-9-18)25-22(27)20-11-19(20)16-5-4-10-23-12-16/h4-10,12-14,19-20H,11H2,1-3H3,(H,25,27)/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged human full-length NAMPT expressed in Escherichia coli Rosetta DE3 using nicotinamide as substrate preincubated fo... |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50448628

(CHEMBL3127503)Show SMILES Fc1cc(F)cc(c1)S(=O)(=O)c1ccc(NC(=O)[C@@H]2C[C@H]2c2cccnc2)cc1 |r| Show InChI InChI=1S/C21H16F2N2O3S/c22-14-8-15(23)10-18(9-14)29(27,28)17-5-3-16(4-6-17)25-21(26)20-11-19(20)13-2-1-7-24-12-13/h1-10,12,19-20H,11H2,(H,25,26)/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged human full-length NAMPT expressed in Escherichia coli Rosetta DE3 using nicotinamide as substrate preincubated fo... |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50448630

(CHEMBL3127499)Show SMILES Cc1ccc(cn1)S(=O)(=O)c1ccc(NC(=O)[C@@H]2C[C@H]2c2cccnc2)cc1 |r| Show InChI InChI=1S/C21H19N3O3S/c1-14-4-7-18(13-23-14)28(26,27)17-8-5-16(6-9-17)24-21(25)20-11-19(20)15-3-2-10-22-12-15/h2-10,12-13,19-20H,11H2,1H3,(H,24,25)/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged human full-length NAMPT expressed in Escherichia coli Rosetta DE3 using nicotinamide as substrate preincubated fo... |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50448638
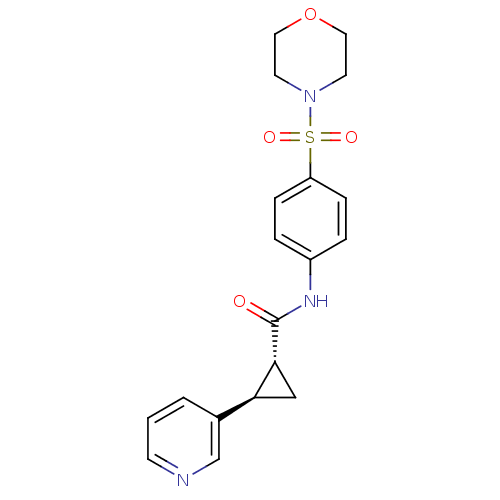
(CHEMBL3127524)Show SMILES O=C(Nc1ccc(cc1)S(=O)(=O)N1CCOCC1)[C@@H]1C[C@H]1c1cccnc1 |r| Show InChI InChI=1S/C19H21N3O4S/c23-19(18-12-17(18)14-2-1-7-20-13-14)21-15-3-5-16(6-4-15)27(24,25)22-8-10-26-11-9-22/h1-7,13,17-18H,8-12H2,(H,21,23)/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged human full-length NAMPT expressed in Escherichia coli Rosetta DE3 using nicotinamide as substrate preincubated fo... |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50448644
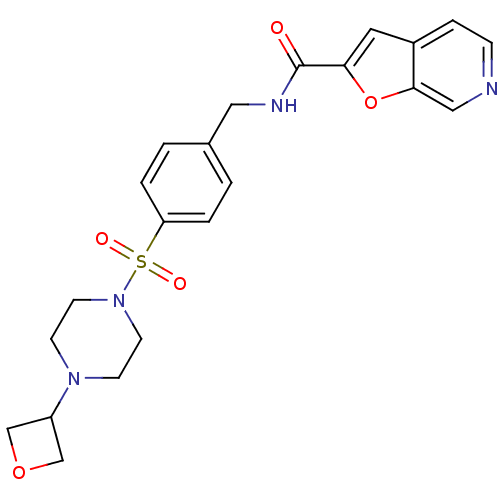
(CHEMBL3127513 | US10696692, Example 149)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)N1CCN(CC1)C1COC1)c1cc2ccncc2o1 Show InChI InChI=1S/C22H24N4O5S/c27-22(20-11-17-5-6-23-13-21(17)31-20)24-12-16-1-3-19(4-2-16)32(28,29)26-9-7-25(8-10-26)18-14-30-15-18/h1-6,11,13,18H,7-10,12,14-15H2,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged human full-length NAMPT expressed in Escherichia coli Rosetta DE3 using nicotinamide as substrate preincubated fo... |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
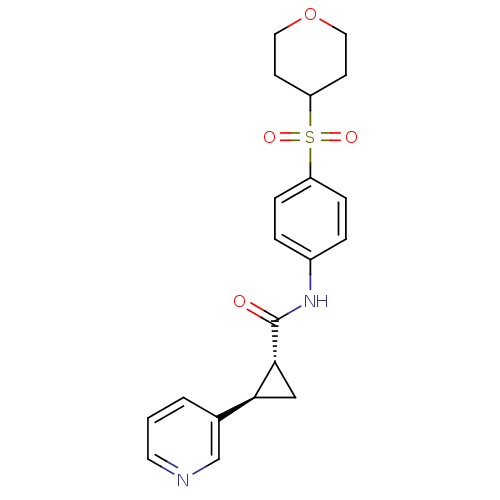
(Homo sapiens (Human)) | BDBM50448618
(CHEMBL3127526)Show SMILES O=C(Nc1ccc(cc1)S(=O)(=O)C1CCOCC1)[C@@H]1C[C@H]1c1cccnc1 |r| Show InChI InChI=1S/C20H22N2O4S/c23-20(19-12-18(19)14-2-1-9-21-13-14)22-15-3-5-16(6-4-15)27(24,25)17-7-10-26-11-8-17/h1-6,9,13,17-19H,7-8,10-12H2,(H,22,23)/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged human full-length NAMPT expressed in Escherichia coli Rosetta DE3 using nicotinamide as substrate preincubated fo... |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50448620
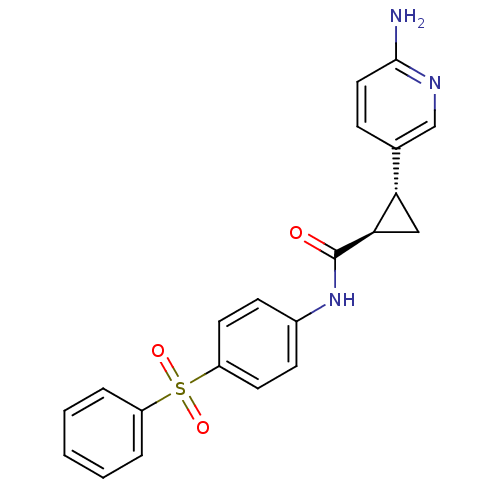
(CHEMBL3127522)Show SMILES Nc1ccc(cn1)[C@@H]1C[C@H]1C(=O)Nc1ccc(cc1)S(=O)(=O)c1ccccc1 |r| Show InChI InChI=1S/C21H19N3O3S/c22-20-11-6-14(13-23-20)18-12-19(18)21(25)24-15-7-9-17(10-8-15)28(26,27)16-4-2-1-3-5-16/h1-11,13,18-19H,12H2,(H2,22,23)(H,24,25)/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged human full-length NAMPT expressed in Escherichia coli Rosetta DE3 using nicotinamide as substrate preincubated fo... |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50448642
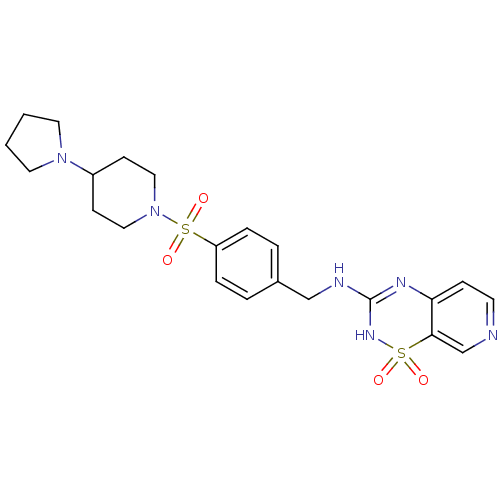
(CHEMBL3127515)Show SMILES O=S(=O)(N1CCC(CC1)N1CCCC1)c1ccc(CNC2=Nc3ccncc3S(=O)(=O)N2)cc1 |t:22| Show InChI InChI=1S/C22H28N6O4S2/c29-33(30)21-16-23-10-7-20(21)25-22(26-33)24-15-17-3-5-19(6-4-17)34(31,32)28-13-8-18(9-14-28)27-11-1-2-12-27/h3-7,10,16,18H,1-2,8-9,11-15H2,(H2,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged human full-length NAMPT expressed in Escherichia coli Rosetta DE3 using nicotinamide as substrate preincubated fo... |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50448643

(CHEMBL3127514)Show SMILES O=S(=O)(c1ccccc1)c1ccc(CNC2=Nc3ccncc3S(=O)(=O)N2)cc1 |t:16| Show InChI InChI=1S/C19H16N4O4S2/c24-28(25,15-4-2-1-3-5-15)16-8-6-14(7-9-16)12-21-19-22-17-10-11-20-13-18(17)29(26,27)23-19/h1-11,13H,12H2,(H2,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged human full-length NAMPT expressed in Escherichia coli Rosetta DE3 using nicotinamide as substrate preincubated fo... |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50448616

(CHEMBL3127501)Show SMILES FC(F)(F)Oc1cccc(c1)S(=O)(=O)c1ccc(NC(=O)[C@@H]2C[C@H]2c2cccnc2)cc1 |r| Show InChI InChI=1S/C22H17F3N2O4S/c23-22(24,25)31-16-4-1-5-18(11-16)32(29,30)17-8-6-15(7-9-17)27-21(28)20-12-19(20)14-3-2-10-26-13-14/h1-11,13,19-20H,12H2,(H,27,28)/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C9 in human liver microsomes using (S)- warfarin as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50448613

(CHEMBL3127509)Show SMILES CC(C)n1cc(cn1)S(=O)(=O)c1ccc(NC(=O)[C@@H]2C[C@H]2c2cccnc2)cc1 |r| Show InChI InChI=1S/C21H22N4O3S/c1-14(2)25-13-18(12-23-25)29(27,28)17-7-5-16(6-8-17)24-21(26)20-10-19(20)15-4-3-9-22-11-15/h3-9,11-14,19-20H,10H2,1-2H3,(H,24,26)/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged human full-length NAMPT expressed in Escherichia coli Rosetta DE3 using nicotinamide as substrate preincubated fo... |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50448613

(CHEMBL3127509)Show SMILES CC(C)n1cc(cn1)S(=O)(=O)c1ccc(NC(=O)[C@@H]2C[C@H]2c2cccnc2)cc1 |r| Show InChI InChI=1S/C21H22N4O3S/c1-14(2)25-13-18(12-23-25)29(27,28)17-7-5-16(6-8-17)24-21(26)20-10-19(20)15-4-3-9-22-11-15/h3-9,11-14,19-20H,10H2,1-2H3,(H,24,26)/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged human full-length NAMPT expressed in Escherichia coli Rosetta DE3 using nicotinamide as substrate preincubated fo... |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50448622

(CHEMBL3127520)Show SMILES O=C(Nc1ccc(cc1)S(=O)(=O)c1ccccc1)[C@@H]1C[C@H]1c1cccnc1 |r| Show InChI InChI=1S/C21H18N2O3S/c24-21(20-13-19(20)15-5-4-12-22-14-15)23-16-8-10-18(11-9-16)27(25,26)17-6-2-1-3-7-17/h1-12,14,19-20H,13H2,(H,23,24)/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C9 in human liver microsomes using (S)- warfarin as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50448617

(CHEMBL3127500)Show SMILES CS(=O)(=O)c1cccc(c1)S(=O)(=O)c1ccc(NC(=O)[C@@H]2C[C@H]2c2cccnc2)cc1 |r| Show InChI InChI=1S/C22H20N2O5S2/c1-30(26,27)18-5-2-6-19(12-18)31(28,29)17-9-7-16(8-10-17)24-22(25)21-13-20(21)15-4-3-11-23-14-15/h2-12,14,20-21H,13H2,1H3,(H,24,25)/t20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C9 in human liver microsomes using (S)- warfarin as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50448621

(CHEMBL3127521)Show SMILES O=C(Nc1ccc(cc1)S(=O)(=O)c1ccccc1)[C@H]1C[C@@H]1c1cccnc1 |r| Show InChI InChI=1S/C21H18N2O3S/c24-21(20-13-19(20)15-5-4-12-22-14-15)23-16-8-10-18(11-9-16)27(25,26)17-6-2-1-3-7-17/h1-12,14,19-20H,13H2,(H,23,24)/t19-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C9 in human liver microsomes using (S)- warfarin as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50448645
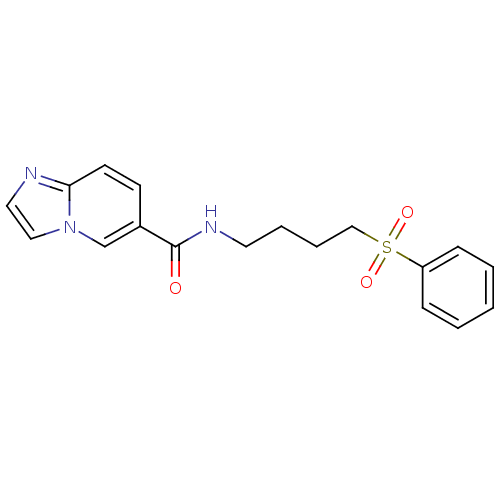
(CHEMBL3127512)Show InChI InChI=1S/C18H19N3O3S/c22-18(15-8-9-17-19-11-12-21(17)14-15)20-10-4-5-13-25(23,24)16-6-2-1-3-7-16/h1-3,6-9,11-12,14H,4-5,10,13H2,(H,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged human full-length NAMPT expressed in Escherichia coli Rosetta DE3 using nicotinamide as substrate preincubated fo... |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50448612

(CHEMBL3127510)Show SMILES CC(C)n1cc(cn1)S(=O)(=O)c1ccc(NC(=O)[C@H]2C[C@@H]2c2cccnc2)cc1 |r| Show InChI InChI=1S/C21H22N4O3S/c1-14(2)25-13-18(12-23-25)29(27,28)17-7-5-16(6-8-17)24-21(26)20-10-19(20)15-4-3-9-22-11-15/h3-9,11-14,19-20H,10H2,1-2H3,(H,24,26)/t19-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C9 in human liver microsomes using (S)- warfarin as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50448615

(CHEMBL3127506)Show SMILES CCCn1cc(cn1)S(=O)(=O)c1ccc(NC(=O)[C@@H]2C[C@H]2c2cccnc2)cc1 |r| Show InChI InChI=1S/C21H22N4O3S/c1-2-10-25-14-18(13-23-25)29(27,28)17-7-5-16(6-8-17)24-21(26)20-11-19(20)15-4-3-9-22-12-15/h3-9,12-14,19-20H,2,10-11H2,1H3,(H,24,26)/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C9 in human liver microsomes using (S)- warfarin as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50448611

(CHEMBL3127494)Show SMILES O=C(Nc1ccc(cc1)S(=O)(=O)c1cnn(c1)C1CCOCC1)[C@@H]1C[C@H]1c1cccnc1 |r| Show InChI InChI=1S/C23H24N4O4S/c28-23(22-12-21(22)16-2-1-9-24-13-16)26-17-3-5-19(6-4-17)32(29,30)20-14-25-27(15-20)18-7-10-31-11-8-18/h1-6,9,13-15,18,21-22H,7-8,10-12H2,(H,26,28)/t21-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C9 in human liver microsomes using (S)- warfarin as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50448622

(CHEMBL3127520)Show SMILES O=C(Nc1ccc(cc1)S(=O)(=O)c1ccccc1)[C@@H]1C[C@H]1c1cccnc1 |r| Show InChI InChI=1S/C21H18N2O3S/c24-21(20-13-19(20)15-5-4-12-22-14-15)23-16-8-10-18(11-9-16)27(25,26)17-6-2-1-3-7-17/h1-12,14,19-20H,13H2,(H,23,24)/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged human full-length NAMPT expressed in Escherichia coli Rosetta DE3 using nicotinamide as substrate preincubated fo... |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50448622

(CHEMBL3127520)Show SMILES O=C(Nc1ccc(cc1)S(=O)(=O)c1ccccc1)[C@@H]1C[C@H]1c1cccnc1 |r| Show InChI InChI=1S/C21H18N2O3S/c24-21(20-13-19(20)15-5-4-12-22-14-15)23-16-8-10-18(11-9-16)27(25,26)17-6-2-1-3-7-17/h1-12,14,19-20H,13H2,(H,23,24)/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged human full-length NAMPT expressed in Escherichia coli Rosetta DE3 using nicotinamide as substrate preincubated fo... |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50448613

(CHEMBL3127509)Show SMILES CC(C)n1cc(cn1)S(=O)(=O)c1ccc(NC(=O)[C@@H]2C[C@H]2c2cccnc2)cc1 |r| Show InChI InChI=1S/C21H22N4O3S/c1-14(2)25-13-18(12-23-25)29(27,28)17-7-5-16(6-8-17)24-21(26)20-10-19(20)15-4-3-9-22-11-15/h3-9,11-14,19-20H,10H2,1-2H3,(H,24,26)/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C9 in human liver microsomes using (S)- warfarin as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50448639
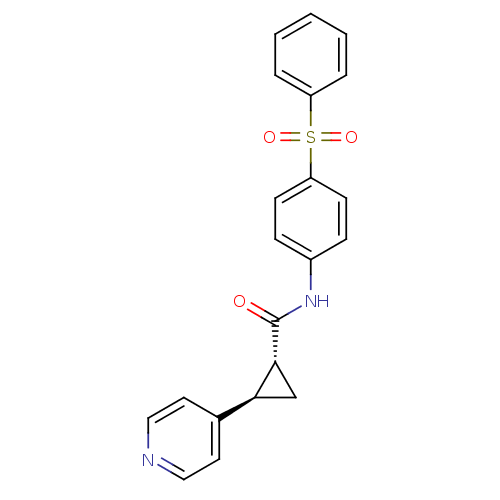
(CHEMBL3127523)Show SMILES O=C(Nc1ccc(cc1)S(=O)(=O)c1ccccc1)[C@@H]1C[C@H]1c1ccncc1 |r| Show InChI InChI=1S/C21H18N2O3S/c24-21(20-14-19(20)15-10-12-22-13-11-15)23-16-6-8-18(9-7-16)27(25,26)17-4-2-1-3-5-17/h1-13,19-20H,14H2,(H,23,24)/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged human full-length NAMPT expressed in Escherichia coli Rosetta DE3 using nicotinamide as substrate preincubated fo... |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50448637

(CHEMBL3127527)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)c1ccccc1)[C@@H]1C[C@H]1c1cccnc1 |r| Show InChI InChI=1S/C22H20N2O3S/c25-22(21-13-20(21)17-5-4-12-23-15-17)24-14-16-8-10-19(11-9-16)28(26,27)18-6-2-1-3-7-18/h1-12,15,20-21H,13-14H2,(H,24,25)/t20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged human full-length NAMPT expressed in Escherichia coli Rosetta DE3 using nicotinamide as substrate preincubated fo... |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50448614

(CHEMBL3127507)Show SMILES CCCn1cc(cn1)S(=O)(=O)c1ccc(NC(=O)[C@H]2C[C@@H]2c2cccnc2)cc1 |r| Show InChI InChI=1S/C21H22N4O3S/c1-2-10-25-14-18(13-23-25)29(27,28)17-7-5-16(6-8-17)24-21(26)20-11-19(20)15-4-3-9-22-12-15/h3-9,12-14,19-20H,2,10-11H2,1H3,(H,24,26)/t19-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C9 in human liver microsomes using (S)- warfarin as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50448640

(CHEMBL3127519)Show InChI InChI=1S/C15H14N2O/c18-15(17-12-6-8-16-9-7-12)14-10-13(14)11-4-2-1-3-5-11/h1-9,13-14H,10H2,(H,16,17,18)/t13-,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged human full-length NAMPT expressed in Escherichia coli Rosetta DE3 using nicotinamide as substrate preincubated fo... |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50448641
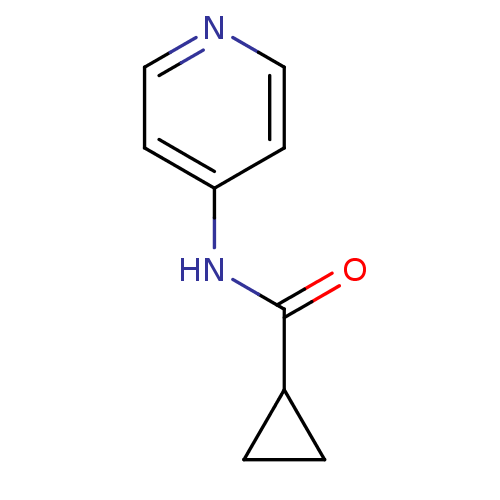
(CHEMBL3127518)Show InChI InChI=1S/C9H10N2O/c12-9(7-1-2-7)11-8-3-5-10-6-4-8/h3-7H,1-2H2,(H,10,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged human full-length NAMPT expressed in Escherichia coli Rosetta DE3 using nicotinamide as substrate preincubated fo... |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50448635

(CHEMBL3127529)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)c1ccccc1)[C@@H]1C[C@H]1c1ccncc1 |r| Show InChI InChI=1S/C22H20N2O3S/c25-22(21-14-20(21)17-10-12-23-13-11-17)24-15-16-6-8-19(9-7-16)28(26,27)18-4-2-1-3-5-18/h1-13,20-21H,14-15H2,(H,24,25)/t20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged human full-length NAMPT expressed in Escherichia coli Rosetta DE3 using nicotinamide as substrate preincubated fo... |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50448648
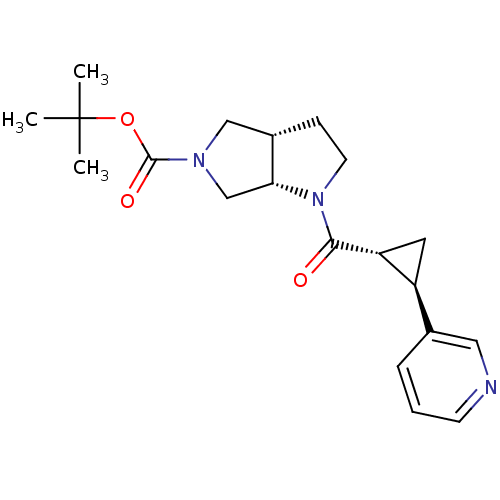
(CHEMBL3127492)Show SMILES CC(C)(C)OC(=O)N1C[C@@H]2CCN([C@@H]2C1)C(=O)[C@@H]1C[C@H]1c1cccnc1 |r| Show InChI InChI=1S/C20H27N3O3/c1-20(2,3)26-19(25)22-11-14-6-8-23(17(14)12-22)18(24)16-9-15(16)13-5-4-7-21-10-13/h4-5,7,10,14-17H,6,8-9,11-12H2,1-3H3/t14-,15-,16+,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged human full-length NAMPT expressed in Escherichia coli Rosetta DE3 using nicotinamide as substrate preincubated fo... |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50448634
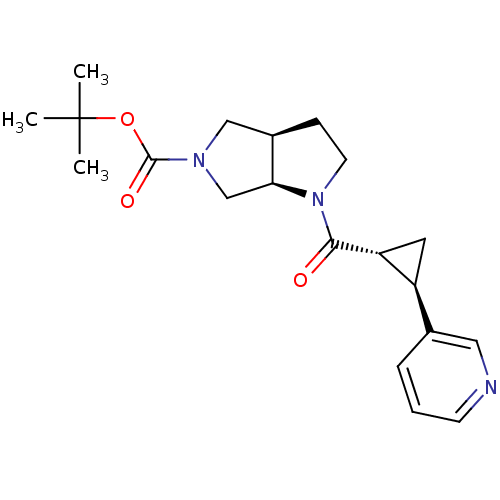
(CHEMBL3127495)Show SMILES CC(C)(C)OC(=O)N1C[C@H]2CCN([C@H]2C1)C(=O)[C@@H]1C[C@H]1c1cccnc1 |r| Show InChI InChI=1S/C20H27N3O3/c1-20(2,3)26-19(25)22-11-14-6-8-23(17(14)12-22)18(24)16-9-15(16)13-5-4-7-21-10-13/h4-5,7,10,14-17H,6,8-9,11-12H2,1-3H3/t14-,15+,16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged human full-length NAMPT expressed in Escherichia coli Rosetta DE3 using nicotinamide as substrate preincubated fo... |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50448633
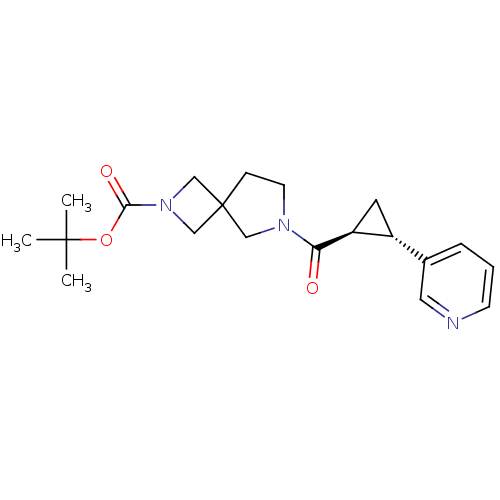
(CHEMBL3127496)Show SMILES CC(C)(C)OC(=O)N1CC2(C1)CCN(C2)C(=O)[C@H]1C[C@@H]1c1cccnc1 |r| Show InChI InChI=1S/C20H27N3O3/c1-19(2,3)26-18(25)23-12-20(13-23)6-8-22(11-20)17(24)16-9-15(16)14-5-4-7-21-10-14/h4-5,7,10,15-16H,6,8-9,11-13H2,1-3H3/t15-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged human full-length NAMPT expressed in Escherichia coli Rosetta DE3 using nicotinamide as substrate preincubated fo... |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50448636

(CHEMBL3127528)Show SMILES Nc1ccc(cn1)[C@@H]1C[C@H]1C(=O)NCc1ccc(cc1)S(=O)(=O)c1ccccc1 |r| Show InChI InChI=1S/C22H21N3O3S/c23-21-11-8-16(14-24-21)19-12-20(19)22(26)25-13-15-6-9-18(10-7-15)29(27,28)17-4-2-1-3-5-17/h1-11,14,19-20H,12-13H2,(H2,23,24)(H,25,26)/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged human full-length NAMPT expressed in Escherichia coli Rosetta DE3 using nicotinamide as substrate preincubated fo... |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50448616

(CHEMBL3127501)Show SMILES FC(F)(F)Oc1cccc(c1)S(=O)(=O)c1ccc(NC(=O)[C@@H]2C[C@H]2c2cccnc2)cc1 |r| Show InChI InChI=1S/C22H17F3N2O4S/c23-22(24,25)31-16-4-1-5-18(11-16)32(29,30)17-8-6-15(7-9-17)27-21(28)20-12-19(20)14-3-2-10-26-13-14/h1-11,13,19-20H,12H2,(H,27,28)/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C19 in human liver microsomes using (S)-mephenytoin as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50448616

(CHEMBL3127501)Show SMILES FC(F)(F)Oc1cccc(c1)S(=O)(=O)c1ccc(NC(=O)[C@@H]2C[C@H]2c2cccnc2)cc1 |r| Show InChI InChI=1S/C22H17F3N2O4S/c23-22(24,25)31-16-4-1-5-18(11-16)32(29,30)17-8-6-15(7-9-17)27-21(28)20-12-19(20)14-3-2-10-26-13-14/h1-11,13,19-20H,12H2,(H,27,28)/t19-,20+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50448622

(CHEMBL3127520)Show SMILES O=C(Nc1ccc(cc1)S(=O)(=O)c1ccccc1)[C@@H]1C[C@H]1c1cccnc1 |r| Show InChI InChI=1S/C21H18N2O3S/c24-21(20-13-19(20)15-5-4-12-22-14-15)23-16-8-10-18(11-9-16)27(25,26)17-6-2-1-3-7-17/h1-12,14,19-20H,13H2,(H,23,24)/t19-,20+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50448619

(CHEMBL3127525)Show SMILES O=C(Nc1ccc(cc1)S(=O)(=O)N1CC2CCC(C1)O2)[C@@H]1C[C@H]1c1cccnc1 |r| Show InChI InChI=1S/C21H23N3O4S/c25-21(20-10-19(20)14-2-1-9-22-11-14)23-15-3-7-18(8-4-15)29(26,27)24-12-16-5-6-17(13-24)28-16/h1-4,7-9,11,16-17,19-20H,5-6,10,12-13H2,(H,23,25)/t16?,17?,19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C9 in human liver microsomes using (S)- warfarin as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50448617

(CHEMBL3127500)Show SMILES CS(=O)(=O)c1cccc(c1)S(=O)(=O)c1ccc(NC(=O)[C@@H]2C[C@H]2c2cccnc2)cc1 |r| Show InChI InChI=1S/C22H20N2O5S2/c1-30(26,27)18-5-2-6-19(12-18)31(28,29)17-9-7-16(8-10-17)24-22(25)21-13-20(21)15-4-3-11-23-14-15/h2-12,14,20-21H,13H2,1H3,(H,24,25)/t20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50448614

(CHEMBL3127507)Show SMILES CCCn1cc(cn1)S(=O)(=O)c1ccc(NC(=O)[C@H]2C[C@@H]2c2cccnc2)cc1 |r| Show InChI InChI=1S/C21H22N4O3S/c1-2-10-25-14-18(13-23-25)29(27,28)17-7-5-16(6-8-17)24-21(26)20-11-19(20)15-4-3-9-22-12-15/h3-9,12-14,19-20H,2,10-11H2,1H3,(H,24,26)/t19-,20+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50448620

(CHEMBL3127522)Show SMILES Nc1ccc(cn1)[C@@H]1C[C@H]1C(=O)Nc1ccc(cc1)S(=O)(=O)c1ccccc1 |r| Show InChI InChI=1S/C21H19N3O3S/c22-20-11-6-14(13-23-20)18-12-19(18)21(25)24-15-7-9-17(10-8-15)28(26,27)16-4-2-1-3-5-16/h1-11,13,18-19H,12H2,(H2,22,23)(H,24,25)/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C9 in human liver microsomes using (S)- warfarin as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50448622

(CHEMBL3127520)Show SMILES O=C(Nc1ccc(cc1)S(=O)(=O)c1ccccc1)[C@@H]1C[C@H]1c1cccnc1 |r| Show InChI InChI=1S/C21H18N2O3S/c24-21(20-13-19(20)15-5-4-12-22-14-15)23-16-8-10-18(11-9-16)27(25,26)17-6-2-1-3-7-17/h1-12,14,19-20H,13H2,(H,23,24)/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50448619

(CHEMBL3127525)Show SMILES O=C(Nc1ccc(cc1)S(=O)(=O)N1CC2CCC(C1)O2)[C@@H]1C[C@H]1c1cccnc1 |r| Show InChI InChI=1S/C21H23N3O4S/c25-21(20-10-19(20)14-2-1-9-22-11-14)23-15-3-7-18(8-4-15)29(26,27)24-12-16-5-6-17(13-24)28-16/h1-4,7-9,11,16-17,19-20H,5-6,10,12-13H2,(H,23,25)/t16?,17?,19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C19 in human liver microsomes using (S)-mephenytoin as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50448622

(CHEMBL3127520)Show SMILES O=C(Nc1ccc(cc1)S(=O)(=O)c1ccccc1)[C@@H]1C[C@H]1c1cccnc1 |r| Show InChI InChI=1S/C21H18N2O3S/c24-21(20-13-19(20)15-5-4-12-22-14-15)23-16-8-10-18(11-9-16)27(25,26)17-6-2-1-3-7-17/h1-12,14,19-20H,13H2,(H,23,24)/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C19 in human liver microsomes using (S)-mephenytoin as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50448611

(CHEMBL3127494)Show SMILES O=C(Nc1ccc(cc1)S(=O)(=O)c1cnn(c1)C1CCOCC1)[C@@H]1C[C@H]1c1cccnc1 |r| Show InChI InChI=1S/C23H24N4O4S/c28-23(22-12-21(22)16-2-1-9-24-13-16)26-17-3-5-19(6-4-17)32(29,30)20-14-25-27(15-20)18-7-10-31-11-8-18/h1-6,9,13-15,18,21-22H,7-8,10-12H2,(H,26,28)/t21-,22+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50448618

(CHEMBL3127526)Show SMILES O=C(Nc1ccc(cc1)S(=O)(=O)C1CCOCC1)[C@@H]1C[C@H]1c1cccnc1 |r| Show InChI InChI=1S/C20H22N2O4S/c23-20(19-12-18(19)14-2-1-9-21-13-14)22-15-3-5-16(6-4-15)27(24,25)17-7-10-26-11-8-17/h1-6,9,13,17-19H,7-8,10-12H2,(H,22,23)/t18-,19+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50448619

(CHEMBL3127525)Show SMILES O=C(Nc1ccc(cc1)S(=O)(=O)N1CC2CCC(C1)O2)[C@@H]1C[C@H]1c1cccnc1 |r| Show InChI InChI=1S/C21H23N3O4S/c25-21(20-10-19(20)14-2-1-9-22-11-14)23-15-3-7-18(8-4-15)29(26,27)24-12-16-5-6-17(13-24)28-16/h1-4,7-9,11,16-17,19-20H,5-6,10,12-13H2,(H,23,25)/t16?,17?,19-,20+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50448614

(CHEMBL3127507)Show SMILES CCCn1cc(cn1)S(=O)(=O)c1ccc(NC(=O)[C@H]2C[C@@H]2c2cccnc2)cc1 |r| Show InChI InChI=1S/C21H22N4O3S/c1-2-10-25-14-18(13-23-25)29(27,28)17-7-5-16(6-8-17)24-21(26)20-11-19(20)15-4-3-9-22-12-15/h3-9,12-14,19-20H,2,10-11H2,1H3,(H,24,26)/t19-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C19 in human liver microsomes using (S)-mephenytoin as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50448621

(CHEMBL3127521)Show SMILES O=C(Nc1ccc(cc1)S(=O)(=O)c1ccccc1)[C@H]1C[C@@H]1c1cccnc1 |r| Show InChI InChI=1S/C21H18N2O3S/c24-21(20-13-19(20)15-5-4-12-22-14-15)23-16-8-10-18(11-9-16)27(25,26)17-6-2-1-3-7-17/h1-12,14,19-20H,13H2,(H,23,24)/t19-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50448618

(CHEMBL3127526)Show SMILES O=C(Nc1ccc(cc1)S(=O)(=O)C1CCOCC1)[C@@H]1C[C@H]1c1cccnc1 |r| Show InChI InChI=1S/C20H22N2O4S/c23-20(19-12-18(19)14-2-1-9-21-13-14)22-15-3-5-16(6-4-15)27(24,25)17-7-10-26-11-8-17/h1-6,9,13,17-19H,7-8,10-12H2,(H,22,23)/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50448615

(CHEMBL3127506)Show SMILES CCCn1cc(cn1)S(=O)(=O)c1ccc(NC(=O)[C@@H]2C[C@H]2c2cccnc2)cc1 |r| Show InChI InChI=1S/C21H22N4O3S/c1-2-10-25-14-18(13-23-25)29(27,28)17-7-5-16(6-8-17)24-21(26)20-11-19(20)15-4-3-9-22-12-15/h3-9,12-14,19-20H,2,10-11H2,1H3,(H,24,26)/t19-,20+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50448617

(CHEMBL3127500)Show SMILES CS(=O)(=O)c1cccc(c1)S(=O)(=O)c1ccc(NC(=O)[C@@H]2C[C@H]2c2cccnc2)cc1 |r| Show InChI InChI=1S/C22H20N2O5S2/c1-30(26,27)18-5-2-6-19(12-18)31(28,29)17-9-7-16(8-10-17)24-22(25)21-13-20(21)15-4-3-11-23-14-15/h2-12,14,20-21H,13H2,1H3,(H,24,25)/t20-,21+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50448612

(CHEMBL3127510)Show SMILES CC(C)n1cc(cn1)S(=O)(=O)c1ccc(NC(=O)[C@H]2C[C@@H]2c2cccnc2)cc1 |r| Show InChI InChI=1S/C21H22N4O3S/c1-14(2)25-13-18(12-23-25)29(27,28)17-7-5-16(6-8-17)24-21(26)20-10-19(20)15-4-3-9-22-11-15/h3-9,11-14,19-20H,10H2,1-2H3,(H,24,26)/t19-,20+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50448615

(CHEMBL3127506)Show SMILES CCCn1cc(cn1)S(=O)(=O)c1ccc(NC(=O)[C@@H]2C[C@H]2c2cccnc2)cc1 |r| Show InChI InChI=1S/C21H22N4O3S/c1-2-10-25-14-18(13-23-25)29(27,28)17-7-5-16(6-8-17)24-21(26)20-11-19(20)15-4-3-9-22-12-15/h3-9,12-14,19-20H,2,10-11H2,1H3,(H,24,26)/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C19 in human liver microsomes using (S)-mephenytoin as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50448619

(CHEMBL3127525)Show SMILES O=C(Nc1ccc(cc1)S(=O)(=O)N1CC2CCC(C1)O2)[C@@H]1C[C@H]1c1cccnc1 |r| Show InChI InChI=1S/C21H23N3O4S/c25-21(20-10-19(20)14-2-1-9-22-11-14)23-15-3-7-18(8-4-15)29(26,27)24-12-16-5-6-17(13-24)28-16/h1-4,7-9,11,16-17,19-20H,5-6,10,12-13H2,(H,23,25)/t16?,17?,19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50448616

(CHEMBL3127501)Show SMILES FC(F)(F)Oc1cccc(c1)S(=O)(=O)c1ccc(NC(=O)[C@@H]2C[C@H]2c2cccnc2)cc1 |r| Show InChI InChI=1S/C22H17F3N2O4S/c23-22(24,25)31-16-4-1-5-18(11-16)32(29,30)17-8-6-15(7-9-17)27-21(28)20-12-19(20)14-3-2-10-26-13-14/h1-11,13,19-20H,12H2,(H,27,28)/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50448617

(CHEMBL3127500)Show SMILES CS(=O)(=O)c1cccc(c1)S(=O)(=O)c1ccc(NC(=O)[C@@H]2C[C@H]2c2cccnc2)cc1 |r| Show InChI InChI=1S/C22H20N2O5S2/c1-30(26,27)18-5-2-6-19(12-18)31(28,29)17-9-7-16(8-10-17)24-22(25)21-13-20(21)15-4-3-11-23-14-15/h2-12,14,20-21H,13H2,1H3,(H,24,25)/t20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C19 in human liver microsomes using (S)-mephenytoin as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50448621

(CHEMBL3127521)Show SMILES O=C(Nc1ccc(cc1)S(=O)(=O)c1ccccc1)[C@H]1C[C@@H]1c1cccnc1 |r| Show InChI InChI=1S/C21H18N2O3S/c24-21(20-13-19(20)15-5-4-12-22-14-15)23-16-8-10-18(11-9-16)27(25,26)17-6-2-1-3-7-17/h1-12,14,19-20H,13H2,(H,23,24)/t19-,20+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50448620

(CHEMBL3127522)Show SMILES Nc1ccc(cn1)[C@@H]1C[C@H]1C(=O)Nc1ccc(cc1)S(=O)(=O)c1ccccc1 |r| Show InChI InChI=1S/C21H19N3O3S/c22-20-11-6-14(13-23-20)18-12-19(18)21(25)24-15-7-9-17(10-8-15)28(26,27)16-4-2-1-3-5-16/h1-11,13,18-19H,12H2,(H2,22,23)(H,24,25)/t18-,19+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50448611

(CHEMBL3127494)Show SMILES O=C(Nc1ccc(cc1)S(=O)(=O)c1cnn(c1)C1CCOCC1)[C@@H]1C[C@H]1c1cccnc1 |r| Show InChI InChI=1S/C23H24N4O4S/c28-23(22-12-21(22)16-2-1-9-24-13-16)26-17-3-5-19(6-4-17)32(29,30)20-14-25-27(15-20)18-7-10-31-11-8-18/h1-6,9,13-15,18,21-22H,7-8,10-12H2,(H,26,28)/t21-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C19 in human liver microsomes using (S)-mephenytoin as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50448612

(CHEMBL3127510)Show SMILES CC(C)n1cc(cn1)S(=O)(=O)c1ccc(NC(=O)[C@H]2C[C@@H]2c2cccnc2)cc1 |r| Show InChI InChI=1S/C21H22N4O3S/c1-14(2)25-13-18(12-23-25)29(27,28)17-7-5-16(6-8-17)24-21(26)20-10-19(20)15-4-3-9-22-11-15/h3-9,11-14,19-20H,10H2,1-2H3,(H,24,26)/t19-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C19 in human liver microsomes using (S)-mephenytoin as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50448620

(CHEMBL3127522)Show SMILES Nc1ccc(cn1)[C@@H]1C[C@H]1C(=O)Nc1ccc(cc1)S(=O)(=O)c1ccccc1 |r| Show InChI InChI=1S/C21H19N3O3S/c22-20-11-6-14(13-23-20)18-12-19(18)21(25)24-15-7-9-17(10-8-15)28(26,27)16-4-2-1-3-5-16/h1-11,13,18-19H,12H2,(H2,22,23)(H,24,25)/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C19 in human liver microsomes using (S)-mephenytoin as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50448621

(CHEMBL3127521)Show SMILES O=C(Nc1ccc(cc1)S(=O)(=O)c1ccccc1)[C@H]1C[C@@H]1c1cccnc1 |r| Show InChI InChI=1S/C21H18N2O3S/c24-21(20-13-19(20)15-5-4-12-22-14-15)23-16-8-10-18(11-9-16)27(25,26)17-6-2-1-3-7-17/h1-12,14,19-20H,13H2,(H,23,24)/t19-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C19 in human liver microsomes using (S)-mephenytoin as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50448613

(CHEMBL3127509)Show SMILES CC(C)n1cc(cn1)S(=O)(=O)c1ccc(NC(=O)[C@@H]2C[C@H]2c2cccnc2)cc1 |r| Show InChI InChI=1S/C21H22N4O3S/c1-14(2)25-13-18(12-23-25)29(27,28)17-7-5-16(6-8-17)24-21(26)20-10-19(20)15-4-3-9-22-11-15/h3-9,11-14,19-20H,10H2,1-2H3,(H,24,26)/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50448615

(CHEMBL3127506)Show SMILES CCCn1cc(cn1)S(=O)(=O)c1ccc(NC(=O)[C@@H]2C[C@H]2c2cccnc2)cc1 |r| Show InChI InChI=1S/C21H22N4O3S/c1-2-10-25-14-18(13-23-25)29(27,28)17-7-5-16(6-8-17)24-21(26)20-11-19(20)15-4-3-9-22-12-15/h3-9,12-14,19-20H,2,10-11H2,1H3,(H,24,26)/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50448618

(CHEMBL3127526)Show SMILES O=C(Nc1ccc(cc1)S(=O)(=O)C1CCOCC1)[C@@H]1C[C@H]1c1cccnc1 |r| Show InChI InChI=1S/C20H22N2O4S/c23-20(19-12-18(19)14-2-1-9-21-13-14)22-15-3-5-16(6-4-15)27(24,25)17-7-10-26-11-8-17/h1-6,9,13,17-19H,7-8,10-12H2,(H,22,23)/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C19 in human liver microsomes using (S)-mephenytoin as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50448613

(CHEMBL3127509)Show SMILES CC(C)n1cc(cn1)S(=O)(=O)c1ccc(NC(=O)[C@@H]2C[C@H]2c2cccnc2)cc1 |r| Show InChI InChI=1S/C21H22N4O3S/c1-14(2)25-13-18(12-23-25)29(27,28)17-7-5-16(6-8-17)24-21(26)20-10-19(20)15-4-3-9-22-11-15/h3-9,11-14,19-20H,10H2,1-2H3,(H,24,26)/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C19 in human liver microsomes using (S)-mephenytoin as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50448614

(CHEMBL3127507)Show SMILES CCCn1cc(cn1)S(=O)(=O)c1ccc(NC(=O)[C@H]2C[C@@H]2c2cccnc2)cc1 |r| Show InChI InChI=1S/C21H22N4O3S/c1-2-10-25-14-18(13-23-25)29(27,28)17-7-5-16(6-8-17)24-21(26)20-11-19(20)15-4-3-9-22-12-15/h3-9,12-14,19-20H,2,10-11H2,1H3,(H,24,26)/t19-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50448618

(CHEMBL3127526)Show SMILES O=C(Nc1ccc(cc1)S(=O)(=O)C1CCOCC1)[C@@H]1C[C@H]1c1cccnc1 |r| Show InChI InChI=1S/C20H22N2O4S/c23-20(19-12-18(19)14-2-1-9-21-13-14)22-15-3-5-16(6-4-15)27(24,25)17-7-10-26-11-8-17/h1-6,9,13,17-19H,7-8,10-12H2,(H,22,23)/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C9 in human liver microsomes using (S)- warfarin as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50448613

(CHEMBL3127509)Show SMILES CC(C)n1cc(cn1)S(=O)(=O)c1ccc(NC(=O)[C@@H]2C[C@H]2c2cccnc2)cc1 |r| Show InChI InChI=1S/C21H22N4O3S/c1-14(2)25-13-18(12-23-25)29(27,28)17-7-5-16(6-8-17)24-21(26)20-10-19(20)15-4-3-9-22-11-15/h3-9,11-14,19-20H,10H2,1-2H3,(H,24,26)/t19-,20+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50448611

(CHEMBL3127494)Show SMILES O=C(Nc1ccc(cc1)S(=O)(=O)c1cnn(c1)C1CCOCC1)[C@@H]1C[C@H]1c1cccnc1 |r| Show InChI InChI=1S/C23H24N4O4S/c28-23(22-12-21(22)16-2-1-9-24-13-16)26-17-3-5-19(6-4-17)32(29,30)20-14-25-27(15-20)18-7-10-31-11-8-18/h1-6,9,13-15,18,21-22H,7-8,10-12H2,(H,26,28)/t21-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50448612

(CHEMBL3127510)Show SMILES CC(C)n1cc(cn1)S(=O)(=O)c1ccc(NC(=O)[C@H]2C[C@@H]2c2cccnc2)cc1 |r| Show InChI InChI=1S/C21H22N4O3S/c1-14(2)25-13-18(12-23-25)29(27,28)17-7-5-16(6-8-17)24-21(26)20-10-19(20)15-4-3-9-22-11-15/h3-9,11-14,19-20H,10H2,1-2H3,(H,24,26)/t19-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50448620

(CHEMBL3127522)Show SMILES Nc1ccc(cn1)[C@@H]1C[C@H]1C(=O)Nc1ccc(cc1)S(=O)(=O)c1ccccc1 |r| Show InChI InChI=1S/C21H19N3O3S/c22-20-11-6-14(13-23-20)18-12-19(18)21(25)24-15-7-9-17(10-8-15)28(26,27)16-4-2-1-3-5-16/h1-11,13,18-19H,12H2,(H2,22,23)(H,24,25)/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate by LC-MS/MS analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50448644

(CHEMBL3127513 | US10696692, Example 149)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)N1CCN(CC1)C1COC1)c1cc2ccncc2o1 Show InChI InChI=1S/C22H24N4O5S/c27-22(20-11-17-5-6-23-13-21(17)31-20)24-12-16-1-3-19(4-2-16)32(28,29)26-9-7-25(8-10-26)18-14-30-15-18/h1-6,11,13,18H,7-10,12,14-15H2,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to NAMPT (2 to 491) (unknown origin) by surface plasmon resonance analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50448640

(CHEMBL3127519)Show InChI InChI=1S/C15H14N2O/c18-15(17-12-6-8-16-9-7-12)14-10-13(14)11-4-2-1-3-5-11/h1-9,13-14H,10H2,(H,16,17,18)/t13-,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to NAMPT (2 to 491) (unknown origin) by surface plasmon resonance analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50448645

(CHEMBL3127512)Show InChI InChI=1S/C18H19N3O3S/c22-18(15-8-9-17-19-11-12-21(17)14-15)20-10-4-5-13-25(23,24)16-6-2-1-3-7-16/h1-3,6-9,11-12,14H,4-5,10,13H2,(H,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to NAMPT (2 to 491) (unknown origin) by surface plasmon resonance analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50448646
(CHEMBL3127517)Show InChI InChI=1S/C10H10N2O3/c13-10(7-1-2-7)11-8-3-5-9(6-4-8)12(14)15/h3-7H,1-2H2,(H,11,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 5.10E+4 | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to NAMPT (2 to 491) (unknown origin) by surface plasmon resonance analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50448647

(CHEMBL3127516)Show InChI InChI=1S/C12H14N2O2/c1-8(15)13-10-4-6-11(7-5-10)14-12(16)9-2-3-9/h4-7,9H,2-3H2,1H3,(H,13,15)(H,14,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 7.80E+4 | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to NAMPT (2 to 491) (unknown origin) by surface plasmon resonance analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50448641

(CHEMBL3127518)Show InChI InChI=1S/C9H10N2O/c12-9(7-1-2-7)11-8-3-5-10-6-4-8/h3-7H,1-2H2,(H,10,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to NAMPT (2 to 491) (unknown origin) by surface plasmon resonance analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM86248
(CAS_364-98-7 | NSC_3019 | diazoxide)Show InChI InChI=1S/C8H7ClN2O2S/c1-5-10-7-3-2-6(9)4-8(7)14(12,13)11-5/h2-4H,1H3,(H,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | n/a | 2.30E+5 | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to NAMPT (2 to 491) (unknown origin) by surface plasmon resonance analysis |
J Med Chem 57: 770-92 (2014)
Article DOI: 10.1021/jm4015108
BindingDB Entry DOI: 10.7270/Q2BC412B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data