 Found 58 hits of Enzyme Inhibition Constant Data
Found 58 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM50031906
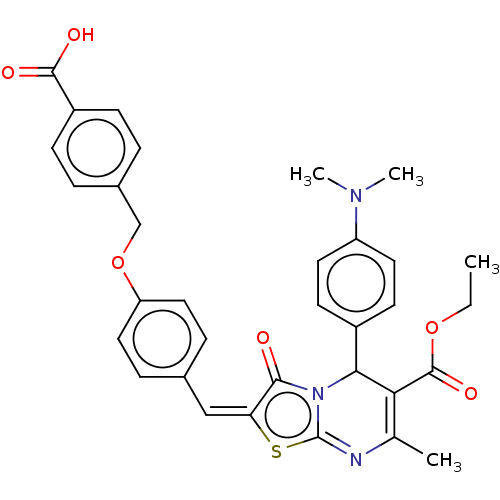
(CHEMBL3360905)Show SMILES CCOC(=O)C1=C(C)N=c2s\c(=C\c3ccc(OCc4ccc(cc4)C(O)=O)cc3)c(=O)n2C1c1ccc(cc1)N(C)C |c:5,t:8| Show InChI InChI=1S/C33H31N3O6S/c1-5-41-32(40)28-20(2)34-33-36(29(28)23-12-14-25(15-13-23)35(3)4)30(37)27(43-33)18-21-8-16-26(17-9-21)42-19-22-6-10-24(11-7-22)31(38)39/h6-18,29H,5,19H2,1-4H3,(H,38,39)/b27-18+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Competitive inhibition of N-terminal His-tagged Lyp (unknown origin) catalytic domain (1 to 294 residues) expressed in Escherichia coli BL21 (DE3) as... |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM50343530
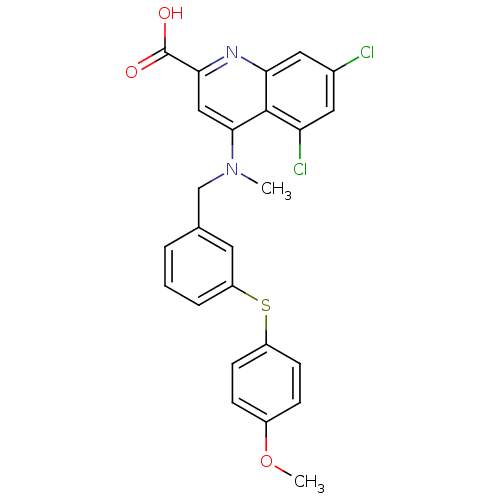
(5,7-dichloro-4-((3-(4-methoxyphenylthio)benzyl)(me...)Show SMILES COc1ccc(Sc2cccc(CN(C)c3cc(nc4cc(Cl)cc(Cl)c34)C(O)=O)c2)cc1 Show InChI InChI=1S/C25H20Cl2N2O3S/c1-29(23-13-22(25(30)31)28-21-12-16(26)11-20(27)24(21)23)14-15-4-3-5-19(10-15)33-18-8-6-17(32-2)7-9-18/h3-13H,14H2,1-2H3,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Competitive inhibition of N-terminal His-tagged Lyp (unknown origin) catalytic domain (1 to 294 residues) expressed in Escherichia coli BL21 (DE3) as... |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM50031908
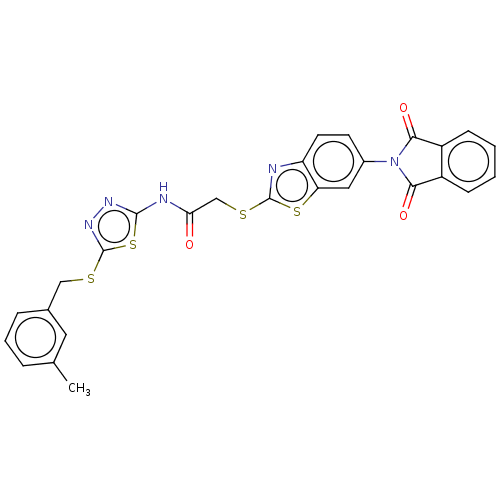
(CHEMBL3360906)Show SMILES Cc1cccc(CSc2nnc(NC(=O)CSc3nc4ccc(cc4s3)N3C(=O)c4ccccc4C3=O)s2)c1 Show InChI InChI=1S/C27H19N5O3S4/c1-15-5-4-6-16(11-15)13-36-27-31-30-25(39-27)29-22(33)14-37-26-28-20-10-9-17(12-21(20)38-26)32-23(34)18-7-2-3-8-19(18)24(32)35/h2-12H,13-14H2,1H3,(H,29,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Competitive inhibition of N-terminal His-tagged Lyp (unknown origin) catalytic domain (1 to 294 residues) expressed in Escherichia coli BL21 (DE3) as... |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM50031910
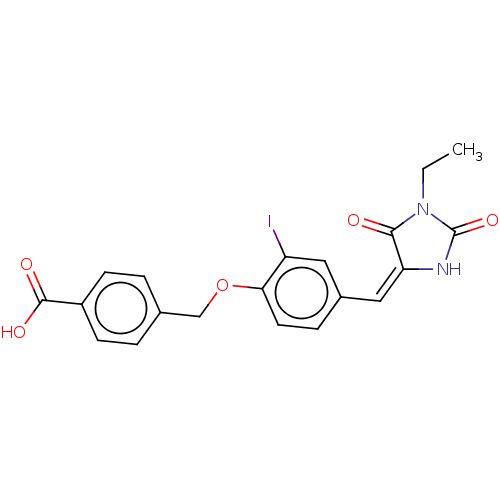
(CHEMBL3360908)Show SMILES CCN1C(=O)N\C(=C\c2ccc(OCc3ccc(cc3)C(O)=O)c(I)c2)C1=O Show InChI InChI=1S/C20H17IN2O5/c1-2-23-18(24)16(22-20(23)27)10-13-5-8-17(15(21)9-13)28-11-12-3-6-14(7-4-12)19(25)26/h3-10H,2,11H2,1H3,(H,22,27)(H,25,26)/b16-10+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Competitive inhibition of N-terminal His-tagged Lyp (unknown origin) catalytic domain (1 to 294 residues) expressed in Escherichia coli BL21 (DE3) as... |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM50031905

(CHEMBL3360904)Show SMILES Brc1ccc(cc1)-c1ccc(o1)C(=O)Nc1ccc2oc(nc2c1)-c1cccnc1 Show InChI InChI=1S/C23H14BrN3O3/c24-16-5-3-14(4-6-16)19-9-10-21(29-19)22(28)26-17-7-8-20-18(12-17)27-23(30-20)15-2-1-11-25-13-15/h1-13H,(H,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Competitive inhibition of N-terminal His-tagged Lyp (unknown origin) catalytic domain (1 to 294 residues) expressed in Escherichia coli BL21 (DE3) as... |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM50031907
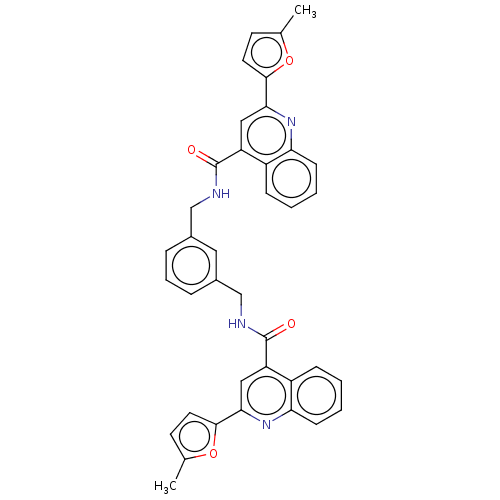
(CHEMBL2337245)Show SMILES Cc1ccc(o1)-c1cc(C(=O)NCc2cccc(CNC(=O)c3cc(nc4ccccc34)-c3ccc(C)o3)c2)c2ccccc2n1 Show InChI InChI=1S/C38H30N4O4/c1-23-14-16-35(45-23)33-19-29(27-10-3-5-12-31(27)41-33)37(43)39-21-25-8-7-9-26(18-25)22-40-38(44)30-20-34(36-17-15-24(2)46-36)42-32-13-6-4-11-28(30)32/h3-20H,21-22H2,1-2H3,(H,39,43)(H,40,44) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Competitive inhibition of N-terminal His-tagged Lyp (unknown origin) catalytic domain (1 to 294 residues) expressed in Escherichia coli BL21 (DE3) as... |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM50031909

(CHEMBL3360907)Show SMILES Cc1c(Cl)cccc1-c1ccc(o1)C(=O)Nc1ccc2C(=O)c3ccccc3C(=O)c2c1 Show InChI InChI=1S/C26H16ClNO4/c1-14-16(7-4-8-21(14)27)22-11-12-23(32-22)26(31)28-15-9-10-19-20(13-15)25(30)18-6-3-2-5-17(18)24(19)29/h2-13H,1H3,(H,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Competitive inhibition of N-terminal His-tagged Lyp (unknown origin) catalytic domain (1 to 294 residues) expressed in Escherichia coli BL21 (DE3) as... |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM50031904
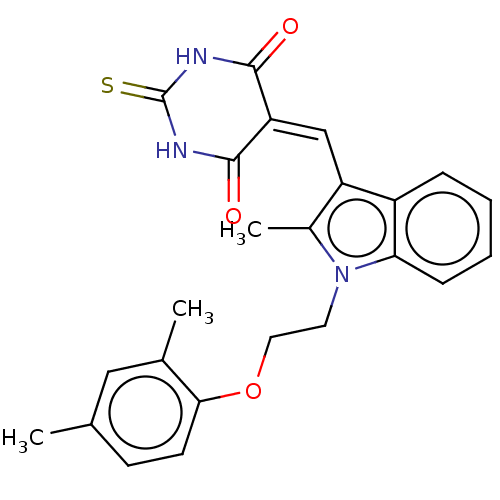
(CHEMBL3360903)Show SMILES [#6]-c1c(\[#6]=[#6]-2\[#6](=O)-[#7]-[#6](=S)-[#7]-[#6]-2=O)c2ccccc2n1-[#6]-[#6]-[#8]-c1ccc(-[#6])cc1-[#6] Show InChI InChI=1S/C24H23N3O3S/c1-14-8-9-21(15(2)12-14)30-11-10-27-16(3)18(17-6-4-5-7-20(17)27)13-19-22(28)25-24(31)26-23(19)29/h4-9,12-13H,10-11H2,1-3H3,(H2,25,26,28,29,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Competitive inhibition of N-terminal His-tagged Lyp (unknown origin) catalytic domain (1 to 294 residues) expressed in Escherichia coli BL21 (DE3) as... |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM50031911

(CHEMBL1299903)Show SMILES CCCCOc1cccc(c1)C1N(Cc2ccco2)C(=O)C(O)=C1C(=O)c1ccc(C)o1 |c:24| Show InChI InChI=1S/C25H25NO6/c1-3-4-12-30-18-8-5-7-17(14-18)22-21(23(27)20-11-10-16(2)32-20)24(28)25(29)26(22)15-19-9-6-13-31-19/h5-11,13-14,22,28H,3-4,12,15H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Covalent irreversible inhibition of N-terminal His-tagged Lyp (unknown origin) catalytic domain (1 to 294 residues) expressed in Escherichia coli BL2... |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50031906

(CHEMBL3360905)Show SMILES CCOC(=O)C1=C(C)N=c2s\c(=C\c3ccc(OCc4ccc(cc4)C(O)=O)cc3)c(=O)n2C1c1ccc(cc1)N(C)C |c:5,t:8| Show InChI InChI=1S/C33H31N3O6S/c1-5-41-32(40)28-20(2)34-33-36(29(28)23-12-14-25(15-13-23)35(3)4)30(37)27(43-33)18-21-8-16-26(17-9-21)42-19-22-6-10-24(11-7-22)31(38)39/h6-18,29H,5,19H2,1-4H3,(H,38,39)/b27-18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) assessed as reduction in pNPP hydrolysis |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM50031906

(CHEMBL3360905)Show SMILES CCOC(=O)C1=C(C)N=c2s\c(=C\c3ccc(OCc4ccc(cc4)C(O)=O)cc3)c(=O)n2C1c1ccc(cc1)N(C)C |c:5,t:8| Show InChI InChI=1S/C33H31N3O6S/c1-5-41-32(40)28-20(2)34-33-36(29(28)23-12-14-25(15-13-23)35(3)4)30(37)27(43-33)18-21-8-16-26(17-9-21)42-19-22-6-10-24(11-7-22)31(38)39/h6-18,29H,5,19H2,1-4H3,(H,38,39)/b27-18+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged Lyp (unknown origin) catalytic domain (1 to 294 residues) expressed in Escherichia coli BL21 (DE3) assessed as re... |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM50031916
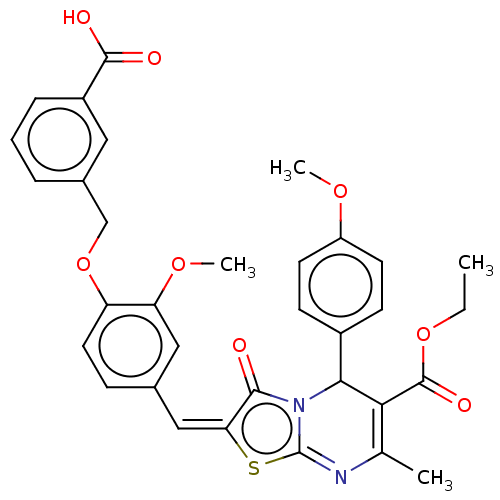
(CHEMBL3360912)Show SMILES CCOC(=O)C1=C(C)N=c2s\c(=C\c3ccc(OCc4cccc(c4)C(O)=O)c(OC)c3)c(=O)n2C1c1ccc(OC)cc1 |c:5,t:8| Show InChI InChI=1S/C33H30N2O8S/c1-5-42-32(39)28-19(2)34-33-35(29(28)22-10-12-24(40-3)13-11-22)30(36)27(44-33)17-20-9-14-25(26(16-20)41-4)43-18-21-7-6-8-23(15-21)31(37)38/h6-17,29H,5,18H2,1-4H3,(H,37,38)/b27-17+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged Lyp (unknown origin) catalytic domain (1 to 294 residues) expressed in Escherichia coli BL21 (DE3) assessed as re... |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50031906

(CHEMBL3360905)Show SMILES CCOC(=O)C1=C(C)N=c2s\c(=C\c3ccc(OCc4ccc(cc4)C(O)=O)cc3)c(=O)n2C1c1ccc(cc1)N(C)C |c:5,t:8| Show InChI InChI=1S/C33H31N3O6S/c1-5-41-32(40)28-20(2)34-33-36(29(28)23-12-14-25(15-13-23)35(3)4)30(37)27(43-33)18-21-8-16-26(17-9-21)42-19-22-6-10-24(11-7-22)31(38)39/h6-18,29H,5,19H2,1-4H3,(H,38,39)/b27-18+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of VHR (unknown origin) assessed as reduction in pNPP hydrolysis |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 9
(Homo sapiens (Human)) | BDBM50031906

(CHEMBL3360905)Show SMILES CCOC(=O)C1=C(C)N=c2s\c(=C\c3ccc(OCc4ccc(cc4)C(O)=O)cc3)c(=O)n2C1c1ccc(cc1)N(C)C |c:5,t:8| Show InChI InChI=1S/C33H31N3O6S/c1-5-41-32(40)28-20(2)34-33-36(29(28)23-12-14-25(15-13-23)35(3)4)30(37)27(43-33)18-21-8-16-26(17-9-21)42-19-22-6-10-24(11-7-22)31(38)39/h6-18,29H,5,19H2,1-4H3,(H,38,39)/b27-18+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of MEG2 (unknown origin) assessed as reduction in pNPP hydrolysis |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50031906

(CHEMBL3360905)Show SMILES CCOC(=O)C1=C(C)N=c2s\c(=C\c3ccc(OCc4ccc(cc4)C(O)=O)cc3)c(=O)n2C1c1ccc(cc1)N(C)C |c:5,t:8| Show InChI InChI=1S/C33H31N3O6S/c1-5-41-32(40)28-20(2)34-33-36(29(28)23-12-14-25(15-13-23)35(3)4)30(37)27(43-33)18-21-8-16-26(17-9-21)42-19-22-6-10-24(11-7-22)31(38)39/h6-18,29H,5,19H2,1-4H3,(H,38,39)/b27-18+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of STEP (unknown origin) assessed as reduction in pNPP hydrolysis |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM50031908

(CHEMBL3360906)Show SMILES Cc1cccc(CSc2nnc(NC(=O)CSc3nc4ccc(cc4s3)N3C(=O)c4ccccc4C3=O)s2)c1 Show InChI InChI=1S/C27H19N5O3S4/c1-15-5-4-6-16(11-15)13-36-27-31-30-25(39-27)29-22(33)14-37-26-28-20-10-9-17(12-21(20)38-26)32-23(34)18-7-2-3-8-19(18)24(32)35/h2-12H,13-14H2,1H3,(H,29,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged Lyp (unknown origin) catalytic domain (1 to 294 residues) expressed in Escherichia coli BL21 (DE3) assessed as re... |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Protein phosphatase 1A
(Homo sapiens (Human)) | BDBM50343530

(5,7-dichloro-4-((3-(4-methoxyphenylthio)benzyl)(me...)Show SMILES COc1ccc(Sc2cccc(CN(C)c3cc(nc4cc(Cl)cc(Cl)c34)C(O)=O)c2)cc1 Show InChI InChI=1S/C25H20Cl2N2O3S/c1-29(23-13-22(25(30)31)28-21-12-16(26)11-20(27)24(21)23)14-15-4-3-5-19(10-15)33-18-8-6-17(32-2)7-9-18/h3-13H,14H2,1-2H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of PPM1A (unknown origin) assessed as reduction in pNPP hydrolysis |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 18
(Homo sapiens (Human)) | BDBM50031906

(CHEMBL3360905)Show SMILES CCOC(=O)C1=C(C)N=c2s\c(=C\c3ccc(OCc4ccc(cc4)C(O)=O)cc3)c(=O)n2C1c1ccc(cc1)N(C)C |c:5,t:8| Show InChI InChI=1S/C33H31N3O6S/c1-5-41-32(40)28-20(2)34-33-36(29(28)23-12-14-25(15-13-23)35(3)4)30(37)27(43-33)18-21-8-16-26(17-9-21)42-19-22-6-10-24(11-7-22)31(38)39/h6-18,29H,5,19H2,1-4H3,(H,38,39)/b27-18+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of PTPN18 (unknown origin) assessed as reduction in pNPP hydrolysis |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM50031920

(CHEMBL3360916)Show SMILES Cc1cccc(CSc2nnc(NC(=O)CSc3nc4ccc(NC(=O)c5ccccc5C)cc4s3)s2)c1 Show InChI InChI=1S/C27H23N5O2S4/c1-16-6-5-8-18(12-16)14-35-27-32-31-25(38-27)30-23(33)15-36-26-29-21-11-10-19(13-22(21)37-26)28-24(34)20-9-4-3-7-17(20)2/h3-13H,14-15H2,1-2H3,(H,28,34)(H,30,31,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged Lyp (unknown origin) catalytic domain (1 to 294 residues) expressed in Escherichia coli BL21 (DE3) assessed as re... |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 18
(Homo sapiens (Human)) | BDBM50031910

(CHEMBL3360908)Show SMILES CCN1C(=O)N\C(=C\c2ccc(OCc3ccc(cc3)C(O)=O)c(I)c2)C1=O Show InChI InChI=1S/C20H17IN2O5/c1-2-23-18(24)16(22-20(23)27)10-13-5-8-17(15(21)9-13)28-11-12-3-6-14(7-4-12)19(25)26/h3-10H,2,11H2,1H3,(H,22,27)(H,25,26)/b16-10+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of PTPN18 (unknown origin) assessed as reduction in pNPP hydrolysis |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Protein phosphatase 1A
(Homo sapiens (Human)) | BDBM50031908

(CHEMBL3360906)Show SMILES Cc1cccc(CSc2nnc(NC(=O)CSc3nc4ccc(cc4s3)N3C(=O)c4ccccc4C3=O)s2)c1 Show InChI InChI=1S/C27H19N5O3S4/c1-15-5-4-6-16(11-15)13-36-27-31-30-25(39-27)29-22(33)14-37-26-28-20-10-9-17(12-21(20)38-26)32-23(34)18-7-2-3-8-19(18)24(32)35/h2-12H,13-14H2,1H3,(H,29,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of PPM1A (unknown origin) assessed as reduction in pNPP hydrolysis |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM50031913

(CHEMBL3360910)Show SMILES CCOC(=O)C1=C(C)N=c2s\c(=C\c3ccc(OCC(O)=O)cc3)c(=O)n2C1c1ccccc1 |c:5,t:8| Show InChI InChI=1S/C25H22N2O6S/c1-3-32-24(31)21-15(2)26-25-27(22(21)17-7-5-4-6-8-17)23(30)19(34-25)13-16-9-11-18(12-10-16)33-14-20(28)29/h4-13,22H,3,14H2,1-2H3,(H,28,29)/b19-13+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged Lyp (unknown origin) catalytic domain (1 to 294 residues) expressed in Escherichia coli BL21 (DE3) assessed as re... |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 9
(Homo sapiens (Human)) | BDBM50031908

(CHEMBL3360906)Show SMILES Cc1cccc(CSc2nnc(NC(=O)CSc3nc4ccc(cc4s3)N3C(=O)c4ccccc4C3=O)s2)c1 Show InChI InChI=1S/C27H19N5O3S4/c1-15-5-4-6-16(11-15)13-36-27-31-30-25(39-27)29-22(33)14-37-26-28-20-10-9-17(12-21(20)38-26)32-23(34)18-7-2-3-8-19(18)24(32)35/h2-12H,13-14H2,1H3,(H,29,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of MEG2 (unknown origin) assessed as reduction in pNPP hydrolysis |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50343530

(5,7-dichloro-4-((3-(4-methoxyphenylthio)benzyl)(me...)Show SMILES COc1ccc(Sc2cccc(CN(C)c3cc(nc4cc(Cl)cc(Cl)c34)C(O)=O)c2)cc1 Show InChI InChI=1S/C25H20Cl2N2O3S/c1-29(23-13-22(25(30)31)28-21-12-16(26)11-20(27)24(21)23)14-15-4-3-5-19(10-15)33-18-8-6-17(32-2)7-9-18/h3-13H,14H2,1-2H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) assessed as reduction in pNPP hydrolysis |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM50031917
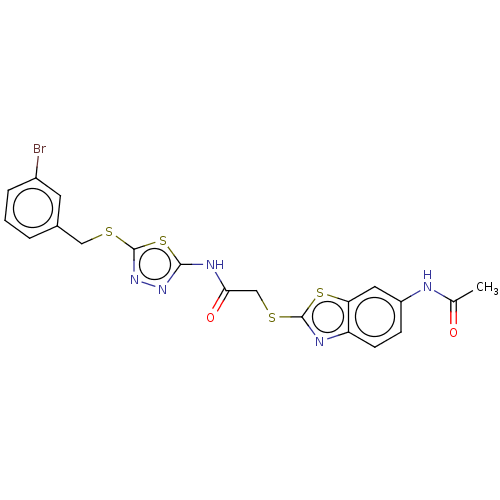
(CHEMBL3360913)Show SMILES CC(=O)Nc1ccc2nc(SCC(=O)Nc3nnc(SCc4cccc(Br)c4)s3)sc2c1 Show InChI InChI=1S/C20H16BrN5O2S4/c1-11(27)22-14-5-6-15-16(8-14)31-19(23-15)30-10-17(28)24-18-25-26-20(32-18)29-9-12-3-2-4-13(21)7-12/h2-8H,9-10H2,1H3,(H,22,27)(H,24,25,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged Lyp (unknown origin) catalytic domain (1 to 294 residues) expressed in Escherichia coli BL21 (DE3) assessed as re... |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM50031919
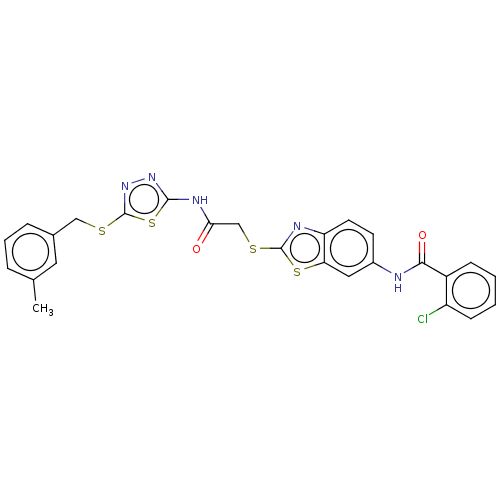
(CHEMBL3360915)Show SMILES Cc1cccc(CSc2nnc(NC(=O)CSc3nc4ccc(NC(=O)c5ccccc5Cl)cc4s3)s2)c1 Show InChI InChI=1S/C26H20ClN5O2S4/c1-15-5-4-6-16(11-15)13-35-26-32-31-24(38-26)30-22(33)14-36-25-29-20-10-9-17(12-21(20)37-25)28-23(34)18-7-2-3-8-19(18)27/h2-12H,13-14H2,1H3,(H,28,34)(H,30,31,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged Lyp (unknown origin) catalytic domain (1 to 294 residues) expressed in Escherichia coli BL21 (DE3) assessed as re... |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM50031918
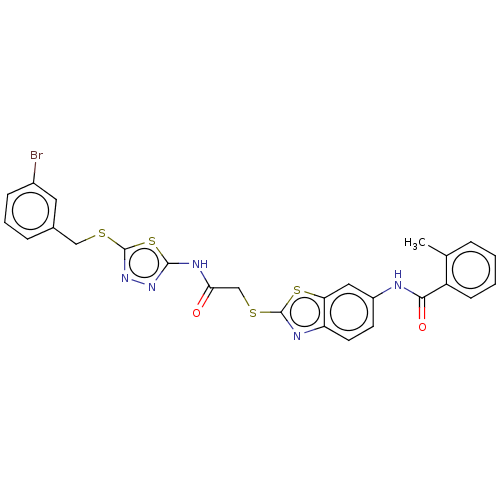
(CHEMBL3360914)Show SMILES Cc1ccccc1C(=O)Nc1ccc2nc(SCC(=O)Nc3nnc(SCc4cccc(Br)c4)s3)sc2c1 Show InChI InChI=1S/C26H20BrN5O2S4/c1-15-5-2-3-8-19(15)23(34)28-18-9-10-20-21(12-18)37-25(29-20)36-14-22(33)30-24-31-32-26(38-24)35-13-16-6-4-7-17(27)11-16/h2-12H,13-14H2,1H3,(H,28,34)(H,30,31,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged Lyp (unknown origin) catalytic domain (1 to 294 residues) expressed in Escherichia coli BL21 (DE3) assessed as re... |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM50031912
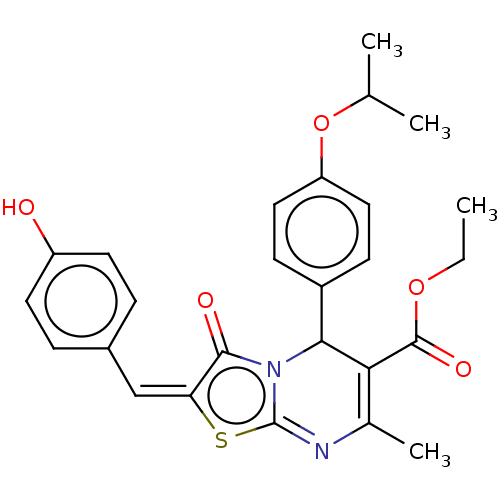
(CHEMBL3360909)Show SMILES CCOC(=O)C1=C(C)N=c2s\c(=C\c3ccc(O)cc3)c(=O)n2C1c1ccc(OC(C)C)cc1 |c:5,t:8| Show InChI InChI=1S/C26H26N2O5S/c1-5-32-25(31)22-16(4)27-26-28(23(22)18-8-12-20(13-9-18)33-15(2)3)24(30)21(34-26)14-17-6-10-19(29)11-7-17/h6-15,23,29H,5H2,1-4H3/b21-14+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged Lyp (unknown origin) catalytic domain (1 to 294 residues) expressed in Escherichia coli BL21 (DE3) assessed as re... |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Protein phosphatase 1A
(Homo sapiens (Human)) | BDBM50031910

(CHEMBL3360908)Show SMILES CCN1C(=O)N\C(=C\c2ccc(OCc3ccc(cc3)C(O)=O)c(I)c2)C1=O Show InChI InChI=1S/C20H17IN2O5/c1-2-23-18(24)16(22-20(23)27)10-13-5-8-17(15(21)9-13)28-11-12-3-6-14(7-4-12)19(25)26/h3-10H,2,11H2,1H3,(H,22,27)(H,25,26)/b16-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of PPM1A (unknown origin) assessed as reduction in pNPP hydrolysis |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM50031946
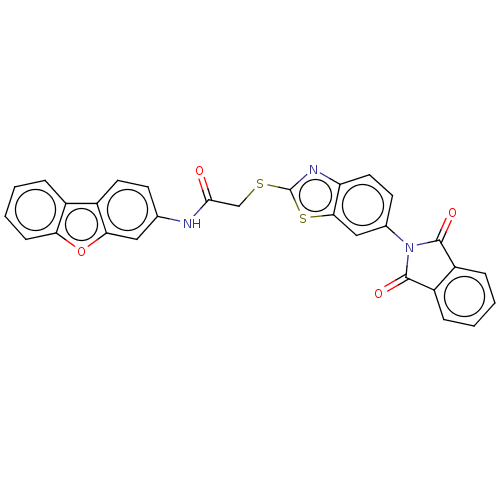
(CHEMBL3360917)Show SMILES O=C(CSc1nc2ccc(cc2s1)N1C(=O)c2ccccc2C1=O)Nc1ccc2c(c1)oc1ccccc21 Show InChI InChI=1S/C29H17N3O4S2/c33-26(30-16-9-11-19-18-5-3-4-8-23(18)36-24(19)13-16)15-37-29-31-22-12-10-17(14-25(22)38-29)32-27(34)20-6-1-2-7-21(20)28(32)35/h1-14H,15H2,(H,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged Lyp (unknown origin) catalytic domain (1 to 294 residues) expressed in Escherichia coli BL21 (DE3) assessed as re... |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50031908

(CHEMBL3360906)Show SMILES Cc1cccc(CSc2nnc(NC(=O)CSc3nc4ccc(cc4s3)N3C(=O)c4ccccc4C3=O)s2)c1 Show InChI InChI=1S/C27H19N5O3S4/c1-15-5-4-6-16(11-15)13-36-27-31-30-25(39-27)29-22(33)14-37-26-28-20-10-9-17(12-21(20)38-26)32-23(34)18-7-2-3-8-19(18)24(32)35/h2-12H,13-14H2,1H3,(H,29,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of VHR (unknown origin) assessed as reduction in pNPP hydrolysis |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50343530

(5,7-dichloro-4-((3-(4-methoxyphenylthio)benzyl)(me...)Show SMILES COc1ccc(Sc2cccc(CN(C)c3cc(nc4cc(Cl)cc(Cl)c34)C(O)=O)c2)cc1 Show InChI InChI=1S/C25H20Cl2N2O3S/c1-29(23-13-22(25(30)31)28-21-12-16(26)11-20(27)24(21)23)14-15-4-3-5-19(10-15)33-18-8-6-17(32-2)7-9-18/h3-13H,14H2,1-2H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of VHR (unknown origin) assessed as reduction in pNPP hydrolysis |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50343530

(5,7-dichloro-4-((3-(4-methoxyphenylthio)benzyl)(me...)Show SMILES COc1ccc(Sc2cccc(CN(C)c3cc(nc4cc(Cl)cc(Cl)c34)C(O)=O)c2)cc1 Show InChI InChI=1S/C25H20Cl2N2O3S/c1-29(23-13-22(25(30)31)28-21-12-16(26)11-20(27)24(21)23)14-15-4-3-5-19(10-15)33-18-8-6-17(32-2)7-9-18/h3-13H,14H2,1-2H3,(H,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of STEP (unknown origin) assessed as reduction in pNPP hydrolysis |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50031910

(CHEMBL3360908)Show SMILES CCN1C(=O)N\C(=C\c2ccc(OCc3ccc(cc3)C(O)=O)c(I)c2)C1=O Show InChI InChI=1S/C20H17IN2O5/c1-2-23-18(24)16(22-20(23)27)10-13-5-8-17(15(21)9-13)28-11-12-3-6-14(7-4-12)19(25)26/h3-10H,2,11H2,1H3,(H,22,27)(H,25,26)/b16-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) assessed as reduction in pNPP hydrolysis |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 9
(Homo sapiens (Human)) | BDBM50343530

(5,7-dichloro-4-((3-(4-methoxyphenylthio)benzyl)(me...)Show SMILES COc1ccc(Sc2cccc(CN(C)c3cc(nc4cc(Cl)cc(Cl)c34)C(O)=O)c2)cc1 Show InChI InChI=1S/C25H20Cl2N2O3S/c1-29(23-13-22(25(30)31)28-21-12-16(26)11-20(27)24(21)23)14-15-4-3-5-19(10-15)33-18-8-6-17(32-2)7-9-18/h3-13H,14H2,1-2H3,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of MEG2 (unknown origin) assessed as reduction in pNPP hydrolysis |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Protein phosphatase 1A
(Homo sapiens (Human)) | BDBM50031906

(CHEMBL3360905)Show SMILES CCOC(=O)C1=C(C)N=c2s\c(=C\c3ccc(OCc4ccc(cc4)C(O)=O)cc3)c(=O)n2C1c1ccc(cc1)N(C)C |c:5,t:8| Show InChI InChI=1S/C33H31N3O6S/c1-5-41-32(40)28-20(2)34-33-36(29(28)23-12-14-25(15-13-23)35(3)4)30(37)27(43-33)18-21-8-16-26(17-9-21)42-19-22-6-10-24(11-7-22)31(38)39/h6-18,29H,5,19H2,1-4H3,(H,38,39)/b27-18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of PPM1A (unknown origin) assessed as reduction in pNPP hydrolysis |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Protein phosphatase 1G
(Homo sapiens (Human)) | BDBM50343530

(5,7-dichloro-4-((3-(4-methoxyphenylthio)benzyl)(me...)Show SMILES COc1ccc(Sc2cccc(CN(C)c3cc(nc4cc(Cl)cc(Cl)c34)C(O)=O)c2)cc1 Show InChI InChI=1S/C25H20Cl2N2O3S/c1-29(23-13-22(25(30)31)28-21-12-16(26)11-20(27)24(21)23)14-15-4-3-5-19(10-15)33-18-8-6-17(32-2)7-9-18/h3-13H,14H2,1-2H3,(H,30,31) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of PPM1G (unknown origin) assessed as reduction in pNPP hydrolysis |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 9
(Homo sapiens (Human)) | BDBM50031910

(CHEMBL3360908)Show SMILES CCN1C(=O)N\C(=C\c2ccc(OCc3ccc(cc3)C(O)=O)c(I)c2)C1=O Show InChI InChI=1S/C20H17IN2O5/c1-2-23-18(24)16(22-20(23)27)10-13-5-8-17(15(21)9-13)28-11-12-3-6-14(7-4-12)19(25)26/h3-10H,2,11H2,1H3,(H,22,27)(H,25,26)/b16-10+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of MEG2 (unknown origin) assessed as reduction in pNPP hydrolysis |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM50031914
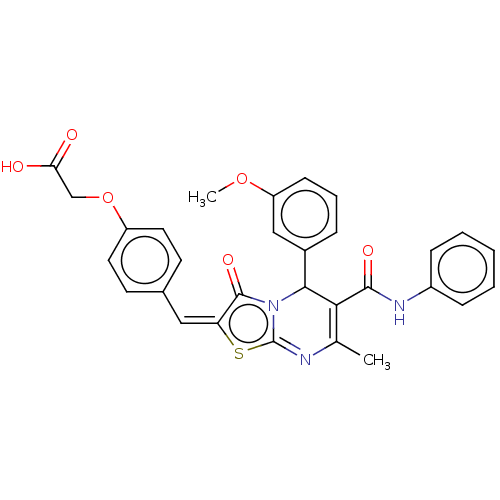
(CHEMBL3196685)Show SMILES COc1cccc(c1)C1C(C(=O)Nc2ccccc2)=C(C)N=c2s\c(=C\c3ccc(OCC(O)=O)cc3)c(=O)n12 |t:20,23| Show InChI InChI=1S/C30H25N3O6S/c1-18-26(28(36)32-21-8-4-3-5-9-21)27(20-7-6-10-23(16-20)38-2)33-29(37)24(40-30(33)31-18)15-19-11-13-22(14-12-19)39-17-25(34)35/h3-16,27H,17H2,1-2H3,(H,32,36)(H,34,35)/b24-15+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged Lyp (unknown origin) catalytic domain (1 to 294 residues) expressed in Escherichia coli BL21 (DE3) assessed as re... |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50031908

(CHEMBL3360906)Show SMILES Cc1cccc(CSc2nnc(NC(=O)CSc3nc4ccc(cc4s3)N3C(=O)c4ccccc4C3=O)s2)c1 Show InChI InChI=1S/C27H19N5O3S4/c1-15-5-4-6-16(11-15)13-36-27-31-30-25(39-27)29-22(33)14-37-26-28-20-10-9-17(12-21(20)38-26)32-23(34)18-7-2-3-8-19(18)24(32)35/h2-12H,13-14H2,1H3,(H,29,30,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of STEP (unknown origin) assessed as reduction in pNPP hydrolysis |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 18
(Homo sapiens (Human)) | BDBM50031908

(CHEMBL3360906)Show SMILES Cc1cccc(CSc2nnc(NC(=O)CSc3nc4ccc(cc4s3)N3C(=O)c4ccccc4C3=O)s2)c1 Show InChI InChI=1S/C27H19N5O3S4/c1-15-5-4-6-16(11-15)13-36-27-31-30-25(39-27)29-22(33)14-37-26-28-20-10-9-17(12-21(20)38-26)32-23(34)18-7-2-3-8-19(18)24(32)35/h2-12H,13-14H2,1H3,(H,29,30,33) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of PTPN18 (unknown origin) assessed as reduction in pNPP hydrolysis |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Protein phosphatase Slingshot homolog 2
(Homo sapiens (Human)) | BDBM50031908

(CHEMBL3360906)Show SMILES Cc1cccc(CSc2nnc(NC(=O)CSc3nc4ccc(cc4s3)N3C(=O)c4ccccc4C3=O)s2)c1 Show InChI InChI=1S/C27H19N5O3S4/c1-15-5-4-6-16(11-15)13-36-27-31-30-25(39-27)29-22(33)14-37-26-28-20-10-9-17(12-21(20)38-26)32-23(34)18-7-2-3-8-19(18)24(32)35/h2-12H,13-14H2,1H3,(H,29,30,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of SSH2 (unknown origin) assessed as reduction in pNPP hydrolysis |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM50031947
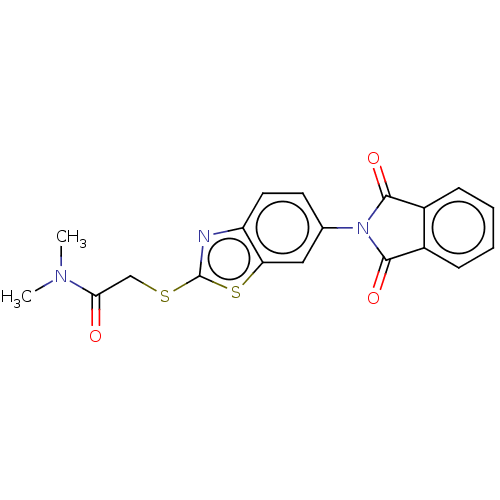
(CHEMBL1363574)Show SMILES CN(C)C(=O)CSc1nc2ccc(cc2s1)N1C(=O)c2ccccc2C1=O Show InChI InChI=1S/C19H15N3O3S2/c1-21(2)16(23)10-26-19-20-14-8-7-11(9-15(14)27-19)22-17(24)12-5-3-4-6-13(12)18(22)25/h3-9H,10H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged Lyp (unknown origin) catalytic domain (1 to 294 residues) expressed in Escherichia coli BL21 (DE3) assessed as re... |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM50031945
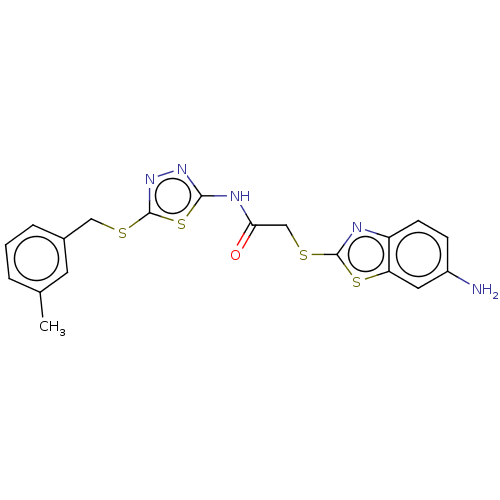
(CHEMBL3352902)Show SMILES Cc1cccc(CSc2nnc(NC(=O)CSc3nc4ccc(N)cc4s3)s2)c1 Show InChI InChI=1S/C19H17N5OS4/c1-11-3-2-4-12(7-11)9-26-19-24-23-17(29-19)22-16(25)10-27-18-21-14-6-5-13(20)8-15(14)28-18/h2-8H,9-10,20H2,1H3,(H,22,23,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged Lyp (unknown origin) catalytic domain (1 to 294 residues) expressed in Escherichia coli BL21 (DE3) assessed as re... |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50031910

(CHEMBL3360908)Show SMILES CCN1C(=O)N\C(=C\c2ccc(OCc3ccc(cc3)C(O)=O)c(I)c2)C1=O Show InChI InChI=1S/C20H17IN2O5/c1-2-23-18(24)16(22-20(23)27)10-13-5-8-17(15(21)9-13)28-11-12-3-6-14(7-4-12)19(25)26/h3-10H,2,11H2,1H3,(H,22,27)(H,25,26)/b16-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of VHR (unknown origin) assessed as reduction in pNPP hydrolysis |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM50032153
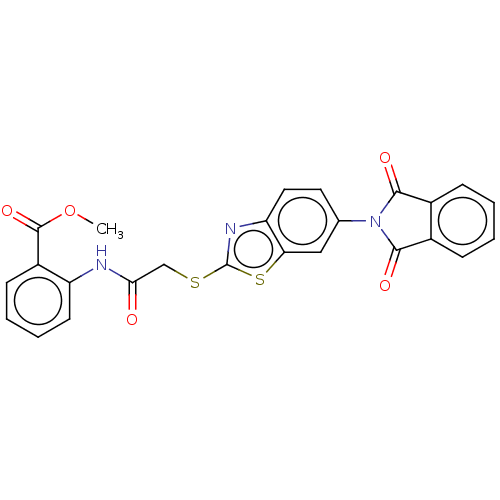
(CHEMBL3360918)Show SMILES COC(=O)c1ccccc1NC(=O)CSc1nc2ccc(cc2s1)N1C(=O)c2ccccc2C1=O Show InChI InChI=1S/C25H17N3O5S2/c1-33-24(32)17-8-4-5-9-18(17)26-21(29)13-34-25-27-19-11-10-14(12-20(19)35-25)28-22(30)15-6-2-3-7-16(15)23(28)31/h2-12H,13H2,1H3,(H,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged Lyp (unknown origin) catalytic domain (1 to 294 residues) expressed in Escherichia coli BL21 (DE3) assessed as re... |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM50031915

(CHEMBL3360911)Show SMILES CCOC(=O)C1=C(C)N=c2s\c(=C\c3ccc(OCc4ccc(cc4)[N+]([O-])=O)c(OCC)c3)c(=O)n2C1c1ccc(OC)c(OC)c1 |c:5,t:8| Show InChI InChI=1S/C34H33N3O9S/c1-6-44-28-16-22(10-14-26(28)46-19-21-8-12-24(13-9-21)37(40)41)17-29-32(38)36-31(23-11-15-25(42-4)27(18-23)43-5)30(33(39)45-7-2)20(3)35-34(36)47-29/h8-18,31H,6-7,19H2,1-5H3/b29-17+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged Lyp (unknown origin) catalytic domain (1 to 294 residues) expressed in Escherichia coli BL21 (DE3) assessed as re... |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Protein phosphatase 1G
(Homo sapiens (Human)) | BDBM50031906

(CHEMBL3360905)Show SMILES CCOC(=O)C1=C(C)N=c2s\c(=C\c3ccc(OCc4ccc(cc4)C(O)=O)cc3)c(=O)n2C1c1ccc(cc1)N(C)C |c:5,t:8| Show InChI InChI=1S/C33H31N3O6S/c1-5-41-32(40)28-20(2)34-33-36(29(28)23-12-14-25(15-13-23)35(3)4)30(37)27(43-33)18-21-8-16-26(17-9-21)42-19-22-6-10-24(11-7-22)31(38)39/h6-18,29H,5,19H2,1-4H3,(H,38,39)/b27-18+ | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of PPM1G (unknown origin) assessed as reduction in pNPP hydrolysis |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Protein phosphatase 1G
(Homo sapiens (Human)) | BDBM50031908

(CHEMBL3360906)Show SMILES Cc1cccc(CSc2nnc(NC(=O)CSc3nc4ccc(cc4s3)N3C(=O)c4ccccc4C3=O)s2)c1 Show InChI InChI=1S/C27H19N5O3S4/c1-15-5-4-6-16(11-15)13-36-27-31-30-25(39-27)29-22(33)14-37-26-28-20-10-9-17(12-21(20)38-26)32-23(34)18-7-2-3-8-19(18)24(32)35/h2-12H,13-14H2,1H3,(H,29,30,33) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of PPM1G (unknown origin) assessed as reduction in pNPP hydrolysis |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Protein phosphatase Slingshot homolog 2
(Homo sapiens (Human)) | BDBM50343530

(5,7-dichloro-4-((3-(4-methoxyphenylthio)benzyl)(me...)Show SMILES COc1ccc(Sc2cccc(CN(C)c3cc(nc4cc(Cl)cc(Cl)c34)C(O)=O)c2)cc1 Show InChI InChI=1S/C25H20Cl2N2O3S/c1-29(23-13-22(25(30)31)28-21-12-16(26)11-20(27)24(21)23)14-15-4-3-5-19(10-15)33-18-8-6-17(32-2)7-9-18/h3-13H,14H2,1-2H3,(H,30,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of SSH2 (unknown origin) assessed as reduction in pNPP hydrolysis |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM50032156
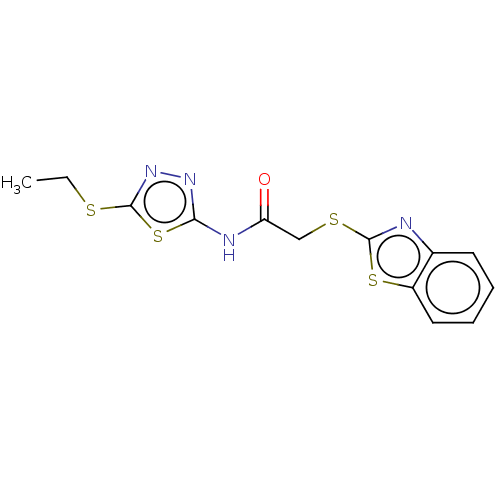
(CHEMBL3360919)Show InChI InChI=1S/C13H12N4OS4/c1-2-19-13-17-16-11(22-13)15-10(18)7-20-12-14-8-5-3-4-6-9(8)21-12/h3-6H,2,7H2,1H3,(H,15,16,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged Lyp (unknown origin) catalytic domain (1 to 294 residues) expressed in Escherichia coli BL21 (DE3) assessed as re... |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Protein phosphatase 1G
(Homo sapiens (Human)) | BDBM50031910

(CHEMBL3360908)Show SMILES CCN1C(=O)N\C(=C\c2ccc(OCc3ccc(cc3)C(O)=O)c(I)c2)C1=O Show InChI InChI=1S/C20H17IN2O5/c1-2-23-18(24)16(22-20(23)27)10-13-5-8-17(15(21)9-13)28-11-12-3-6-14(7-4-12)19(25)26/h3-10H,2,11H2,1H3,(H,22,27)(H,25,26)/b16-10+ | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of PPM1G (unknown origin) assessed as reduction in pNPP hydrolysis |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Protein phosphatase Slingshot homolog 2
(Homo sapiens (Human)) | BDBM50031910

(CHEMBL3360908)Show SMILES CCN1C(=O)N\C(=C\c2ccc(OCc3ccc(cc3)C(O)=O)c(I)c2)C1=O Show InChI InChI=1S/C20H17IN2O5/c1-2-23-18(24)16(22-20(23)27)10-13-5-8-17(15(21)9-13)28-11-12-3-6-14(7-4-12)19(25)26/h3-10H,2,11H2,1H3,(H,22,27)(H,25,26)/b16-10+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of SSH2 (unknown origin) assessed as reduction in pNPP hydrolysis |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Protein phosphatase Slingshot homolog 2
(Homo sapiens (Human)) | BDBM50031906

(CHEMBL3360905)Show SMILES CCOC(=O)C1=C(C)N=c2s\c(=C\c3ccc(OCc4ccc(cc4)C(O)=O)cc3)c(=O)n2C1c1ccc(cc1)N(C)C |c:5,t:8| Show InChI InChI=1S/C33H31N3O6S/c1-5-41-32(40)28-20(2)34-33-36(29(28)23-12-14-25(15-13-23)35(3)4)30(37)27(43-33)18-21-8-16-26(17-9-21)42-19-22-6-10-24(11-7-22)31(38)39/h6-18,29H,5,19H2,1-4H3,(H,38,39)/b27-18+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of SSH2 (unknown origin) assessed as reduction in pNPP hydrolysis |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 18
(Homo sapiens (Human)) | BDBM50343530

(5,7-dichloro-4-((3-(4-methoxyphenylthio)benzyl)(me...)Show SMILES COc1ccc(Sc2cccc(CN(C)c3cc(nc4cc(Cl)cc(Cl)c34)C(O)=O)c2)cc1 Show InChI InChI=1S/C25H20Cl2N2O3S/c1-29(23-13-22(25(30)31)28-21-12-16(26)11-20(27)24(21)23)14-15-4-3-5-19(10-15)33-18-8-6-17(32-2)7-9-18/h3-13H,14H2,1-2H3,(H,30,31) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of PTPN18 (unknown origin) assessed as reduction in pNPP hydrolysis |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50031908

(CHEMBL3360906)Show SMILES Cc1cccc(CSc2nnc(NC(=O)CSc3nc4ccc(cc4s3)N3C(=O)c4ccccc4C3=O)s2)c1 Show InChI InChI=1S/C27H19N5O3S4/c1-15-5-4-6-16(11-15)13-36-27-31-30-25(39-27)29-22(33)14-37-26-28-20-10-9-17(12-21(20)38-26)32-23(34)18-7-2-3-8-19(18)24(32)35/h2-12H,13-14H2,1H3,(H,29,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) assessed as reduction in pNPP hydrolysis |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50031910

(CHEMBL3360908)Show SMILES CCN1C(=O)N\C(=C\c2ccc(OCc3ccc(cc3)C(O)=O)c(I)c2)C1=O Show InChI InChI=1S/C20H17IN2O5/c1-2-23-18(24)16(22-20(23)27)10-13-5-8-17(15(21)9-13)28-11-12-3-6-14(7-4-12)19(25)26/h3-10H,2,11H2,1H3,(H,22,27)(H,25,26)/b16-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of STEP (unknown origin) assessed as reduction in pNPP hydrolysis |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM50031906

(CHEMBL3360905)Show SMILES CCOC(=O)C1=C(C)N=c2s\c(=C\c3ccc(OCc4ccc(cc4)C(O)=O)cc3)c(=O)n2C1c1ccc(cc1)N(C)C |c:5,t:8| Show InChI InChI=1S/C33H31N3O6S/c1-5-41-32(40)28-20(2)34-33-36(29(28)23-12-14-25(15-13-23)35(3)4)30(37)27(43-33)18-21-8-16-26(17-9-21)42-19-22-6-10-24(11-7-22)31(38)39/h6-18,29H,5,19H2,1-4H3,(H,38,39)/b27-18+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.49E+3 | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of Lyp in antiCD3-antibody-stimulated human Jurkat T cells assessed as activation of TCR-mediated and IL-2 promoter driven NFAT/AP1 transc... |
J Med Chem 57: 9309-22 (2014)
Article DOI: 10.1021/jm500692u
BindingDB Entry DOI: 10.7270/Q2ZG6TVJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data