 Found 61 hits of Enzyme Inhibition Constant Data
Found 61 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) using tyrosine 4 as substrate by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50499592

(CHEMBL3741490)Show InChI InChI=1S/C21H21N3O4/c1-3-15-5-4-6-16(11-15)24-21-17-12-19(28-10-9-26-2)20(27-8-7-25)13-18(17)22-14-23-21/h1,4-6,11-14,25H,7-10H2,2H3,(H,22,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) using tyrosine 4 as substrate by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50499586
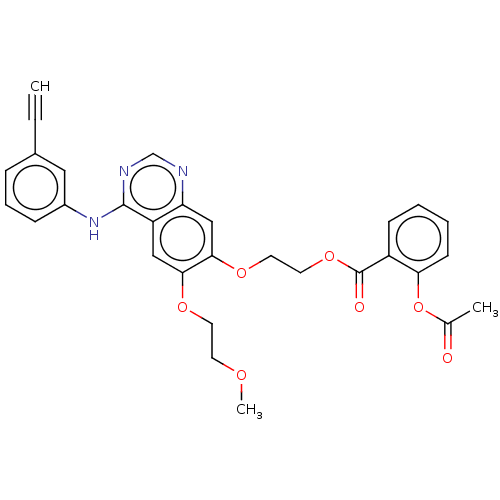
(CHEMBL3741424)Show SMILES COCCOc1cc2c(Nc3cccc(c3)C#C)ncnc2cc1OCCOC(=O)c1ccccc1OC(C)=O Show InChI InChI=1S/C30H27N3O7/c1-4-21-8-7-9-22(16-21)33-29-24-17-27(37-13-12-36-3)28(18-25(24)31-19-32-29)38-14-15-39-30(35)23-10-5-6-11-26(23)40-20(2)34/h1,5-11,16-19H,12-15H2,2-3H3,(H,31,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) using tyrosine 4 as substrate by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50499595
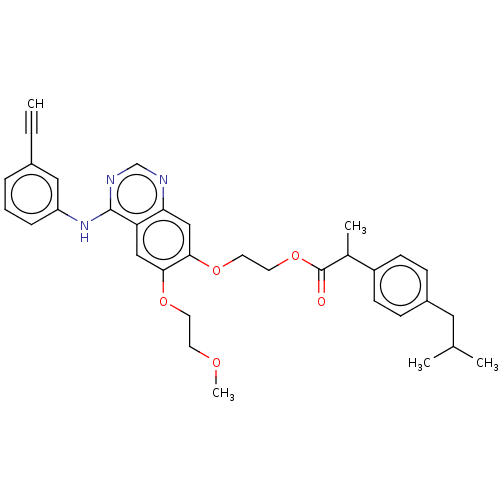
(CHEMBL3741026)Show SMILES COCCOc1cc2c(Nc3cccc(c3)C#C)ncnc2cc1OCCOC(=O)C(C)c1ccc(CC(C)C)cc1 Show InChI InChI=1S/C34H37N3O5/c1-6-25-8-7-9-28(19-25)37-33-29-20-31(40-15-14-39-5)32(21-30(29)35-22-36-33)41-16-17-42-34(38)24(4)27-12-10-26(11-13-27)18-23(2)3/h1,7-13,19-24H,14-18H2,2-5H3,(H,35,36,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) using tyrosine 4 as substrate by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50499589

(CHEMBL3740595)Show SMILES COCCOc1cc2c(Nc3cccc(c3)C#C)ncnc2cc1OCCOC(=O)Cc1c(C)n(C(=O)c2ccc(Cl)cc2)c2ccc(OC)cc12 Show InChI InChI=1S/C40H35ClN4O7/c1-5-26-7-6-8-29(19-26)44-39-33-21-36(50-16-15-48-3)37(23-34(33)42-24-43-39)51-17-18-52-38(46)22-31-25(2)45(35-14-13-30(49-4)20-32(31)35)40(47)27-9-11-28(41)12-10-27/h1,6-14,19-21,23-24H,15-18,22H2,2-4H3,(H,42,43,44) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) using tyrosine 4 as substrate by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50499587
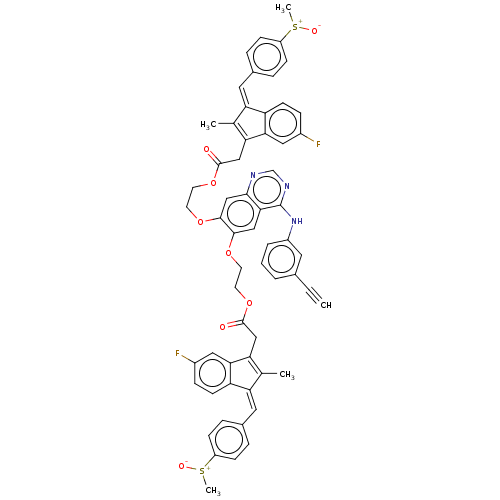
(CHEMBL3739527)Show SMILES C[S+]([O-])c1ccc(\C=C2\C(C)=C(CC(=O)OCCOc3cc4ncnc(Nc5cccc(c5)C#C)c4cc3OCCOC(=O)CC3=C(C)\C(=C\c4ccc(cc4)[S+](C)[O-])c4ccc(F)cc34)c3cc(F)ccc23)cc1 |c:48,t:10| Show InChI InChI=1S/C60H49F2N3O8S2/c1-6-38-8-7-9-43(26-38)65-60-54-31-56(70-22-24-72-58(66)32-50-36(2)48(46-20-14-41(61)29-52(46)50)27-39-10-16-44(17-11-39)74(4)68)57(34-55(54)63-35-64-60)71-23-25-73-59(67)33-51-37(3)49(47-21-15-42(62)30-53(47)51)28-40-12-18-45(19-13-40)75(5)69/h1,7-21,26-31,34-35H,22-25,32-33H2,2-5H3,(H,63,64,65)/b48-27-,49-28- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) using tyrosine 4 as substrate by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50499591
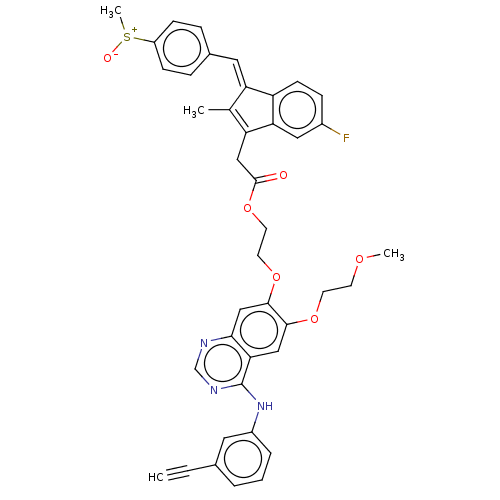
(CHEMBL3742059)Show SMILES COCCOc1cc2c(Nc3cccc(c3)C#C)ncnc2cc1OCCOC(=O)CC1=C(C)\C(=C/c2ccc(cc2)[S+](C)[O-])c2ccc(F)cc12 |c:34| Show InChI InChI=1S/C41H36FN3O6S/c1-5-27-7-6-8-30(19-27)45-41-36-22-38(49-16-15-48-3)39(24-37(36)43-25-44-41)50-17-18-51-40(46)23-34-26(2)33(32-14-11-29(42)21-35(32)34)20-28-9-12-31(13-10-28)52(4)47/h1,6-14,19-22,24-25H,15-18,23H2,2-4H3,(H,43,44,45)/b33-20+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) using tyrosine 4 as substrate by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50499596

(CHEMBL3741304)Show SMILES COCCOc1cc2c(Nc3cccc(c3)C#C)ncnc2cc1OCCOC(=O)c1ccc(cc1)C(=O)Nc1ccc2c(c1)C(C)(C)CCC2(C)C Show InChI InChI=1S/C43H44N4O6/c1-7-28-9-8-10-31(23-28)46-39-33-25-37(51-20-19-50-6)38(26-36(33)44-27-45-39)52-21-22-53-41(49)30-13-11-29(12-14-30)40(48)47-32-15-16-34-35(24-32)43(4,5)18-17-42(34,2)3/h1,8-16,23-27H,17-22H2,2-6H3,(H,47,48)(H,44,45,46) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) using tyrosine 4 as substrate by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50499584

(CHEMBL3739771)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(=O)OCCOc3cc4ncnc(Nc5cccc(c5)C#C)c4cc3OCCOC(=O)Cc3c(C)n(C(=O)c4ccc(Cl)cc4)c4ccc(OC)cc34)c2c1 Show InChI InChI=1S/C58H47Cl2N5O10/c1-6-36-8-7-9-41(26-36)63-56-48-29-52(72-22-24-74-54(66)30-44-34(2)64(50-20-18-42(70-4)27-46(44)50)57(68)37-10-14-39(59)15-11-37)53(32-49(48)61-33-62-56)73-23-25-75-55(67)31-45-35(3)65(51-21-19-43(71-5)28-47(45)51)58(69)38-12-16-40(60)17-13-38/h1,7-21,26-29,32-33H,22-25,30-31H2,2-5H3,(H,61,62,63) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) using tyrosine 4 as substrate by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50499588

(CHEMBL3741399)Show SMILES CC(=O)Oc1ccccc1C(=O)OCCOc1cc2ncnc(Nc3cccc(c3)C#C)c2cc1OCCOC(=O)c1ccccc1OC(C)=O Show InChI InChI=1S/C38H31N3O10/c1-4-26-10-9-11-27(20-26)41-36-30-21-34(46-16-18-48-37(44)28-12-5-7-14-32(28)50-24(2)42)35(22-31(30)39-23-40-36)47-17-19-49-38(45)29-13-6-8-15-33(29)51-25(3)43/h1,5-15,20-23H,16-19H2,2-3H3,(H,39,40,41) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) using tyrosine 4 as substrate by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50022271
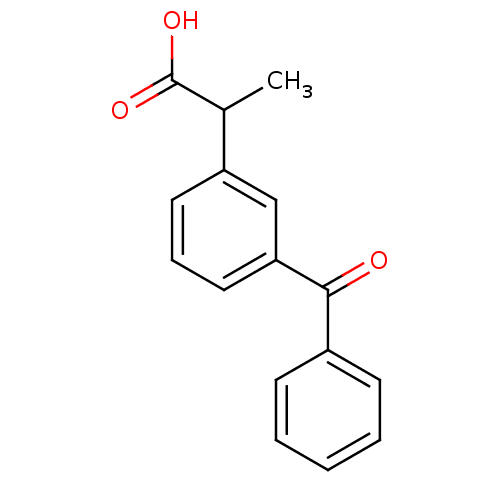
(2-(3-Benzoylphenyl)propionic acid | 2-(3-benzoylph...)Show InChI InChI=1S/C16H14O3/c1-11(16(18)19)13-8-5-9-14(10-13)15(17)12-6-3-2-4-7-12/h2-11H,1H3,(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX-2 preincubated for 15 mins by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50022271

(2-(3-Benzoylphenyl)propionic acid | 2-(3-benzoylph...)Show InChI InChI=1S/C16H14O3/c1-11(16(18)19)13-8-5-9-14(10-13)15(17)12-6-3-2-4-7-12/h2-11H,1H3,(H,18,19) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of Ovine COX-1 preincubated for 15 mins by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50499585
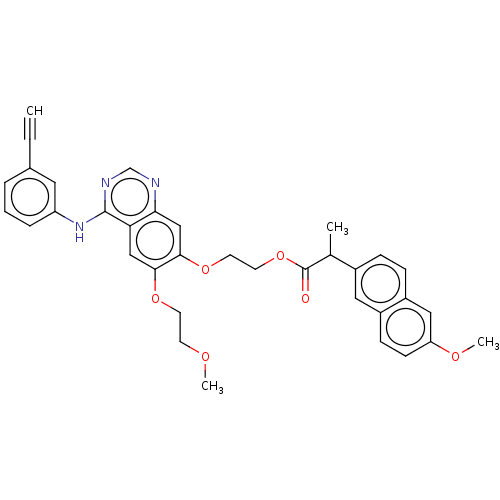
(CHEMBL3740850)Show SMILES COCCOc1cc2c(Nc3cccc(c3)C#C)ncnc2cc1OCCOC(=O)C(C)c1ccc2cc(OC)ccc2c1 Show InChI InChI=1S/C35H33N3O6/c1-5-24-7-6-8-28(17-24)38-34-30-20-32(42-14-13-40-3)33(21-31(30)36-22-37-34)43-15-16-44-35(39)23(2)25-9-10-27-19-29(41-4)12-11-26(27)18-25/h1,6-12,17-23H,13-16H2,2-4H3,(H,36,37,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) using tyrosine 4 as substrate by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of Ovine COX-1 preincubated for 15 mins by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50009860
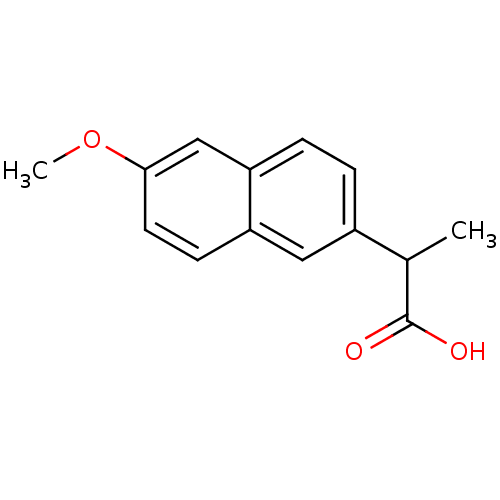
(2-(6-Methoxy-naphthalen-2-yl)-propionic acid | 2-(...)Show InChI InChI=1S/C14H14O3/c1-9(14(15)16)10-3-4-12-8-13(17-2)6-5-11(12)7-10/h3-9H,1-2H3,(H,15,16) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of Ovine COX-1 preincubated for 15 mins by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50009860

(2-(6-Methoxy-naphthalen-2-yl)-propionic acid | 2-(...)Show InChI InChI=1S/C14H14O3/c1-9(14(15)16)10-3-4-12-8-13(17-2)6-5-11(12)7-10/h3-9H,1-2H3,(H,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX-2 preincubated for 15 mins by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50499590
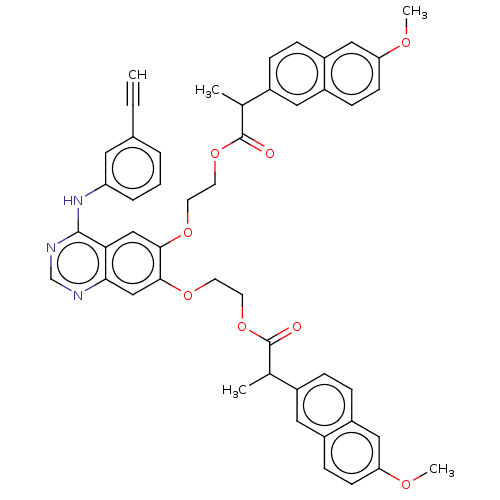
(CHEMBL3741768)Show SMILES COc1ccc2cc(ccc2c1)C(C)C(=O)OCCOc1cc2ncnc(Nc3cccc(c3)C#C)c2cc1OCCOC(=O)C(C)c1ccc2cc(OC)ccc2c1 Show InChI InChI=1S/C48H43N3O8/c1-6-32-8-7-9-39(22-32)51-46-42-27-44(56-18-20-58-47(52)30(2)33-10-12-37-25-40(54-4)16-14-35(37)23-33)45(28-43(42)49-29-50-46)57-19-21-59-48(53)31(3)34-11-13-38-26-41(55-5)17-15-36(38)24-34/h1,7-17,22-31H,18-21H2,2-5H3,(H,49,50,51) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) using tyrosine 4 as substrate by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX-2 preincubated for 15 mins by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50499594
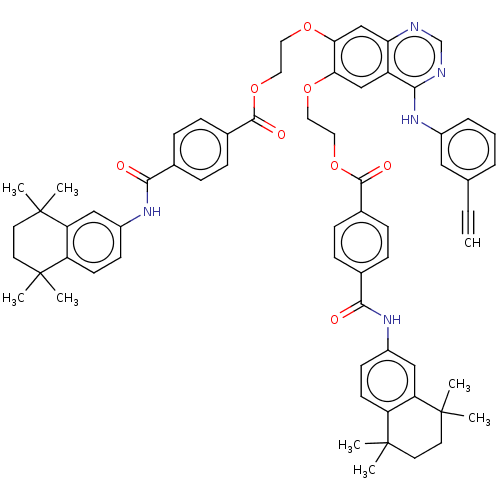
(CHEMBL3740105)Show SMILES CC1(C)CCC(C)(C)c2cc(NC(=O)c3ccc(cc3)C(=O)OCCOc3cc4ncnc(Nc5cccc(c5)C#C)c4cc3OCCOC(=O)c3ccc(cc3)C(=O)Nc3ccc4c(c3)C(C)(C)CCC4(C)C)ccc12 Show InChI InChI=1S/C64H65N5O8/c1-10-40-12-11-13-45(34-40)67-56-48-37-54(74-30-32-76-59(72)43-18-14-41(15-19-43)57(70)68-46-22-24-49-51(35-46)63(6,7)28-26-61(49,2)3)55(38-53(48)65-39-66-56)75-31-33-77-60(73)44-20-16-42(17-21-44)58(71)69-47-23-25-50-52(36-47)64(8,9)29-27-62(50,4)5/h1,11-25,34-39H,26-33H2,2-9H3,(H,68,70)(H,69,71)(H,65,66,67) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) using tyrosine 4 as substrate by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50009859
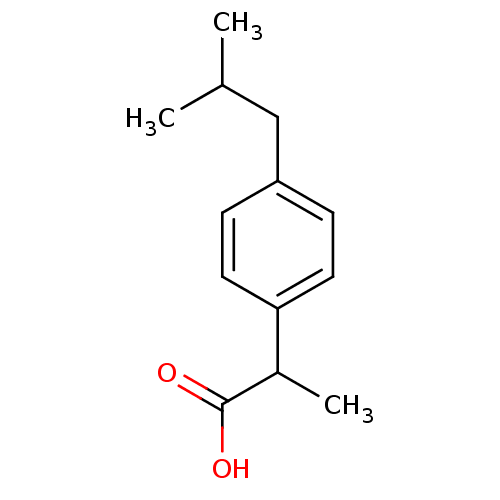
((+-)-2-(p-isobutylphenyl)propionic acid | (+-)-alp...)Show InChI InChI=1S/C13H18O2/c1-9(2)8-11-4-6-12(7-5-11)10(3)13(14)15/h4-7,9-10H,8H2,1-3H3,(H,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX-2 preincubated for 15 mins by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50103504
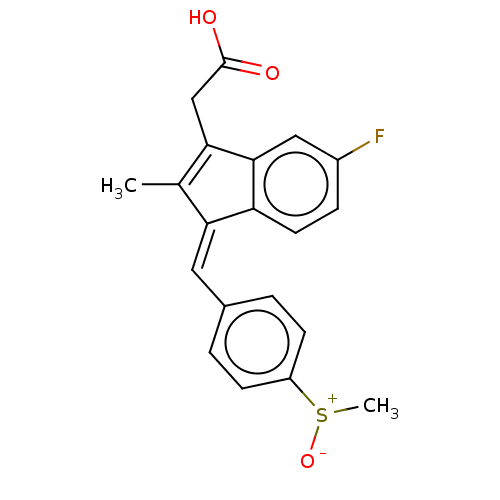
(CHEBI:9352 | Clinoril | Sulindac)Show SMILES C[S+]([O-])c1ccc(\C=C2\C(C)=C(CC(O)=O)c3cc(F)ccc23)cc1 |t:10| Show InChI InChI=1S/C20H17FO3S/c1-12-17(9-13-3-6-15(7-4-13)25(2)24)16-8-5-14(21)10-19(16)18(12)11-20(22)23/h3-10H,11H2,1-2H3,(H,22,23)/b17-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX-2 preincubated for 15 mins by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50499596

(CHEMBL3741304)Show SMILES COCCOc1cc2c(Nc3cccc(c3)C#C)ncnc2cc1OCCOC(=O)c1ccc(cc1)C(=O)Nc1ccc2c(c1)C(C)(C)CCC2(C)C Show InChI InChI=1S/C43H44N4O6/c1-7-28-9-8-10-31(23-28)46-39-33-25-37(51-20-19-50-6)38(26-36(33)44-27-45-39)52-21-22-53-41(49)30-13-11-29(12-14-30)40(48)47-32-15-16-34-35(24-32)43(4,5)18-17-42(34,2)3/h1,8-16,23-27H,17-22H2,2-6H3,(H,47,48)(H,44,45,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX-2 preincubated for 15 mins by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM22360
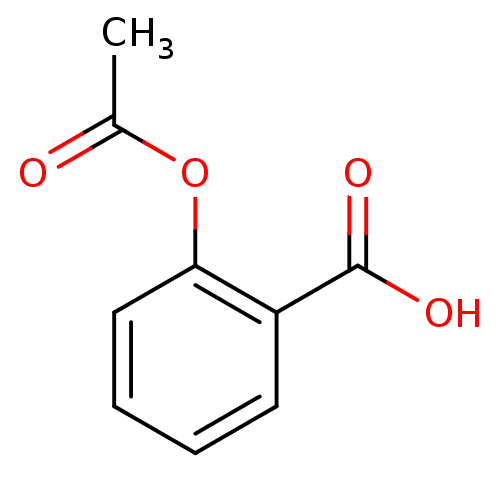
(2-(acetyloxy)benzoate | 2-(acetyloxy)benzoic acid ...)Show InChI InChI=1S/C9H8O4/c1-6(10)13-8-5-3-2-4-7(8)9(11)12/h2-5H,1H3,(H,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX-2 preincubated for 15 mins by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50009859

((+-)-2-(p-isobutylphenyl)propionic acid | (+-)-alp...)Show InChI InChI=1S/C13H18O2/c1-9(2)8-11-4-6-12(7-5-11)10(3)13(14)15/h4-7,9-10H,8H2,1-3H3,(H,14,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) using tyrosine 4 as substrate by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50499591

(CHEMBL3742059)Show SMILES COCCOc1cc2c(Nc3cccc(c3)C#C)ncnc2cc1OCCOC(=O)CC1=C(C)\C(=C/c2ccc(cc2)[S+](C)[O-])c2ccc(F)cc12 |c:34| Show InChI InChI=1S/C41H36FN3O6S/c1-5-27-7-6-8-30(19-27)45-41-36-22-38(49-16-15-48-3)39(24-37(36)43-25-44-41)50-17-18-51-40(46)23-34-26(2)33(32-14-11-29(42)21-35(32)34)20-28-9-12-31(13-10-28)52(4)47/h1,6-14,19-22,24-25H,15-18,23H2,2-4H3,(H,43,44,45)/b33-20+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX-2 preincubated for 15 mins by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50499595

(CHEMBL3741026)Show SMILES COCCOc1cc2c(Nc3cccc(c3)C#C)ncnc2cc1OCCOC(=O)C(C)c1ccc(CC(C)C)cc1 Show InChI InChI=1S/C34H37N3O5/c1-6-25-8-7-9-28(19-25)37-33-29-20-31(40-15-14-39-5)32(21-30(29)35-22-36-33)41-16-17-42-34(38)24(4)27-12-10-26(11-13-27)18-23(2)3/h1,7-13,19-24H,14-18H2,2-5H3,(H,35,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX-2 preincubated for 15 mins by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50499590

(CHEMBL3741768)Show SMILES COc1ccc2cc(ccc2c1)C(C)C(=O)OCCOc1cc2ncnc(Nc3cccc(c3)C#C)c2cc1OCCOC(=O)C(C)c1ccc2cc(OC)ccc2c1 Show InChI InChI=1S/C48H43N3O8/c1-6-32-8-7-9-39(22-32)51-46-42-27-44(56-18-20-58-47(52)30(2)33-10-12-37-25-40(54-4)16-14-35(37)23-33)45(28-43(42)49-29-50-46)57-19-21-59-48(53)31(3)34-11-13-38-26-41(55-5)17-15-36(38)24-34/h1,7-17,22-31H,18-21H2,2-5H3,(H,49,50,51) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX-2 preincubated for 15 mins by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50499584

(CHEMBL3739771)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(=O)OCCOc3cc4ncnc(Nc5cccc(c5)C#C)c4cc3OCCOC(=O)Cc3c(C)n(C(=O)c4ccc(Cl)cc4)c4ccc(OC)cc34)c2c1 Show InChI InChI=1S/C58H47Cl2N5O10/c1-6-36-8-7-9-41(26-36)63-56-48-29-52(72-22-24-74-54(66)30-44-34(2)64(50-20-18-42(70-4)27-46(44)50)57(68)37-10-14-39(59)15-11-37)53(32-49(48)61-33-62-56)73-23-25-75-55(67)31-45-35(3)65(51-21-19-43(71-5)28-47(45)51)58(69)38-12-16-40(60)17-13-38/h1,7-21,26-29,32-33H,22-25,30-31H2,2-5H3,(H,61,62,63) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX-2 preincubated for 15 mins by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50499590

(CHEMBL3741768)Show SMILES COc1ccc2cc(ccc2c1)C(C)C(=O)OCCOc1cc2ncnc(Nc3cccc(c3)C#C)c2cc1OCCOC(=O)C(C)c1ccc2cc(OC)ccc2c1 Show InChI InChI=1S/C48H43N3O8/c1-6-32-8-7-9-39(22-32)51-46-42-27-44(56-18-20-58-47(52)30(2)33-10-12-37-25-40(54-4)16-14-35(37)23-33)45(28-43(42)49-29-50-46)57-19-21-59-48(53)31(3)34-11-13-38-26-41(55-5)17-15-36(38)24-34/h1,7-17,22-31H,18-21H2,2-5H3,(H,49,50,51) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of Ovine COX-1 preincubated for 15 mins by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50009859

((+-)-2-(p-isobutylphenyl)propionic acid | (+-)-alp...)Show InChI InChI=1S/C13H18O2/c1-9(2)8-11-4-6-12(7-5-11)10(3)13(14)15/h4-7,9-10H,8H2,1-3H3,(H,14,15) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of Ovine COX-1 preincubated for 15 mins by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50499585

(CHEMBL3740850)Show SMILES COCCOc1cc2c(Nc3cccc(c3)C#C)ncnc2cc1OCCOC(=O)C(C)c1ccc2cc(OC)ccc2c1 Show InChI InChI=1S/C35H33N3O6/c1-5-24-7-6-8-28(17-24)38-34-30-20-32(42-14-13-40-3)33(21-31(30)36-22-37-34)43-15-16-44-35(39)23(2)25-9-10-27-19-29(41-4)12-11-26(27)18-25/h1,6-12,17-23H,13-16H2,2-4H3,(H,36,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX-2 preincubated for 15 mins by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50103504

(CHEBI:9352 | Clinoril | Sulindac)Show SMILES C[S+]([O-])c1ccc(\C=C2\C(C)=C(CC(O)=O)c3cc(F)ccc23)cc1 |t:10| Show InChI InChI=1S/C20H17FO3S/c1-12-17(9-13-3-6-15(7-4-13)25(2)24)16-8-5-14(21)10-19(16)18(12)11-20(22)23/h3-10H,11H2,1-2H3,(H,22,23)/b17-9- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) using tyrosine 4 as substrate by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50499587

(CHEMBL3739527)Show SMILES C[S+]([O-])c1ccc(\C=C2\C(C)=C(CC(=O)OCCOc3cc4ncnc(Nc5cccc(c5)C#C)c4cc3OCCOC(=O)CC3=C(C)\C(=C\c4ccc(cc4)[S+](C)[O-])c4ccc(F)cc34)c3cc(F)ccc23)cc1 |c:48,t:10| Show InChI InChI=1S/C60H49F2N3O8S2/c1-6-38-8-7-9-43(26-38)65-60-54-31-56(70-22-24-72-58(66)32-50-36(2)48(46-20-14-41(61)29-52(46)50)27-39-10-16-44(17-11-39)74(4)68)57(34-55(54)63-35-64-60)71-23-25-73-59(67)33-51-37(3)49(47-21-15-42(62)30-53(47)51)28-40-12-18-45(19-13-40)75(5)69/h1,7-21,26-31,34-35H,22-25,32-33H2,2-5H3,(H,63,64,65)/b48-27-,49-28- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of Ovine COX-1 preincubated for 15 mins by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50499586

(CHEMBL3741424)Show SMILES COCCOc1cc2c(Nc3cccc(c3)C#C)ncnc2cc1OCCOC(=O)c1ccccc1OC(C)=O Show InChI InChI=1S/C30H27N3O7/c1-4-21-8-7-9-22(16-21)33-29-24-17-27(37-13-12-36-3)28(18-25(24)31-19-32-29)38-14-15-39-30(35)23-10-5-6-11-26(23)40-20(2)34/h1,5-11,16-19H,12-15H2,2-3H3,(H,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX-2 preincubated for 15 mins by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50499589

(CHEMBL3740595)Show SMILES COCCOc1cc2c(Nc3cccc(c3)C#C)ncnc2cc1OCCOC(=O)Cc1c(C)n(C(=O)c2ccc(Cl)cc2)c2ccc(OC)cc12 Show InChI InChI=1S/C40H35ClN4O7/c1-5-26-7-6-8-29(19-26)44-39-33-21-36(50-16-15-48-3)37(23-34(33)42-24-43-39)51-17-18-52-38(46)22-31-25(2)45(35-14-13-30(49-4)20-32(31)35)40(47)27-9-11-28(41)12-10-27/h1,6-14,19-21,23-24H,15-18,22H2,2-4H3,(H,42,43,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX-2 preincubated for 15 mins by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50499588

(CHEMBL3741399)Show SMILES CC(=O)Oc1ccccc1C(=O)OCCOc1cc2ncnc(Nc3cccc(c3)C#C)c2cc1OCCOC(=O)c1ccccc1OC(C)=O Show InChI InChI=1S/C38H31N3O10/c1-4-26-10-9-11-27(20-26)41-36-30-21-34(46-16-18-48-37(44)28-12-5-7-14-32(28)50-24(2)42)35(22-31(30)39-23-40-36)47-17-19-49-38(45)29-13-6-8-15-33(29)51-25(3)43/h1,5-15,20-23H,16-19H2,2-3H3,(H,39,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX-2 preincubated for 15 mins by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) using tyrosine 4 as substrate by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50022271

(2-(3-Benzoylphenyl)propionic acid | 2-(3-benzoylph...)Show InChI InChI=1S/C16H14O3/c1-11(16(18)19)13-8-5-9-14(10-13)15(17)12-6-3-2-4-7-12/h2-11H,1H3,(H,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) using tyrosine 4 as substrate by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50499592

(CHEMBL3741490)Show InChI InChI=1S/C21H21N3O4/c1-3-15-5-4-6-16(11-15)24-21-17-12-19(28-10-9-26-2)20(27-8-7-25)13-18(17)22-14-23-21/h1,4-6,11-14,25H,7-10H2,2H3,(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX-2 preincubated for 15 mins by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50499592

(CHEMBL3741490)Show InChI InChI=1S/C21H21N3O4/c1-3-15-5-4-6-16(11-15)24-21-17-12-19(28-10-9-26-2)20(27-8-7-25)13-18(17)22-14-23-21/h1,4-6,11-14,25H,7-10H2,2H3,(H,22,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of Ovine COX-1 preincubated for 15 mins by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50499593
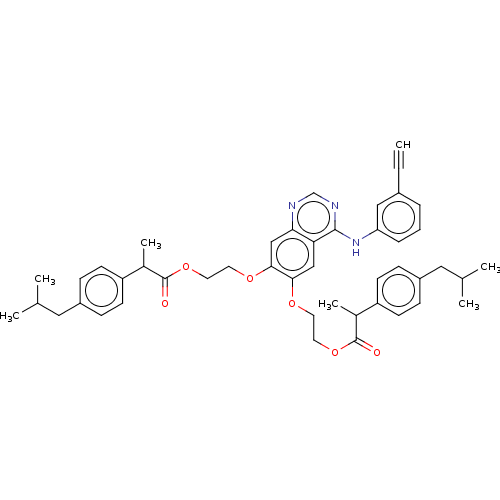
(CHEMBL3739528)Show SMILES CC(C)Cc1ccc(cc1)C(C)C(=O)OCCOc1cc2ncnc(Nc3cccc(c3)C#C)c2cc1OCCOC(=O)C(C)c1ccc(CC(C)C)cc1 Show InChI InChI=1S/C46H51N3O6/c1-8-34-10-9-11-39(26-34)49-44-40-27-42(52-20-22-54-45(50)32(6)37-16-12-35(13-17-37)24-30(2)3)43(28-41(40)47-29-48-44)53-21-23-55-46(51)33(7)38-18-14-36(15-19-38)25-31(4)5/h1,9-19,26-33H,20-25H2,2-7H3,(H,47,48,49) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of Ovine COX-1 preincubated for 15 mins by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50009860

(2-(6-Methoxy-naphthalen-2-yl)-propionic acid | 2-(...)Show InChI InChI=1S/C14H14O3/c1-9(14(15)16)10-3-4-12-8-13(17-2)6-5-11(12)7-10/h3-9H,1-2H3,(H,15,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) using tyrosine 4 as substrate by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50499593

(CHEMBL3739528)Show SMILES CC(C)Cc1ccc(cc1)C(C)C(=O)OCCOc1cc2ncnc(Nc3cccc(c3)C#C)c2cc1OCCOC(=O)C(C)c1ccc(CC(C)C)cc1 Show InChI InChI=1S/C46H51N3O6/c1-8-34-10-9-11-39(26-34)49-44-40-27-42(52-20-22-54-45(50)32(6)37-16-12-35(13-17-37)24-30(2)3)43(28-41(40)47-29-48-44)53-21-23-55-46(51)33(7)38-18-14-36(15-19-38)25-31(4)5/h1,9-19,26-33H,20-25H2,2-7H3,(H,47,48,49) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) using tyrosine 4 as substrate by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50499586

(CHEMBL3741424)Show SMILES COCCOc1cc2c(Nc3cccc(c3)C#C)ncnc2cc1OCCOC(=O)c1ccccc1OC(C)=O Show InChI InChI=1S/C30H27N3O7/c1-4-21-8-7-9-22(16-21)33-29-24-17-27(37-13-12-36-3)28(18-25(24)31-19-32-29)38-14-15-39-30(35)23-10-5-6-11-26(23)40-20(2)34/h1,5-11,16-19H,12-15H2,2-3H3,(H,31,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of Ovine COX-1 preincubated for 15 mins by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50499591

(CHEMBL3742059)Show SMILES COCCOc1cc2c(Nc3cccc(c3)C#C)ncnc2cc1OCCOC(=O)CC1=C(C)\C(=C/c2ccc(cc2)[S+](C)[O-])c2ccc(F)cc12 |c:34| Show InChI InChI=1S/C41H36FN3O6S/c1-5-27-7-6-8-30(19-27)45-41-36-22-38(49-16-15-48-3)39(24-37(36)43-25-44-41)50-17-18-51-40(46)23-34-26(2)33(32-14-11-29(42)21-35(32)34)20-28-9-12-31(13-10-28)52(4)47/h1,6-14,19-22,24-25H,15-18,23H2,2-4H3,(H,43,44,45)/b33-20+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of Ovine COX-1 preincubated for 15 mins by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX-2 preincubated for 15 mins by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50499587

(CHEMBL3739527)Show SMILES C[S+]([O-])c1ccc(\C=C2\C(C)=C(CC(=O)OCCOc3cc4ncnc(Nc5cccc(c5)C#C)c4cc3OCCOC(=O)CC3=C(C)\C(=C\c4ccc(cc4)[S+](C)[O-])c4ccc(F)cc34)c3cc(F)ccc23)cc1 |c:48,t:10| Show InChI InChI=1S/C60H49F2N3O8S2/c1-6-38-8-7-9-43(26-38)65-60-54-31-56(70-22-24-72-58(66)32-50-36(2)48(46-20-14-41(61)29-52(46)50)27-39-10-16-44(17-11-39)74(4)68)57(34-55(54)63-35-64-60)71-23-25-73-59(67)33-51-37(3)49(47-21-15-42(62)30-53(47)51)28-40-12-18-45(19-13-40)75(5)69/h1,7-21,26-31,34-35H,22-25,32-33H2,2-5H3,(H,63,64,65)/b48-27-,49-28- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX-2 preincubated for 15 mins by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50499596

(CHEMBL3741304)Show SMILES COCCOc1cc2c(Nc3cccc(c3)C#C)ncnc2cc1OCCOC(=O)c1ccc(cc1)C(=O)Nc1ccc2c(c1)C(C)(C)CCC2(C)C Show InChI InChI=1S/C43H44N4O6/c1-7-28-9-8-10-31(23-28)46-39-33-25-37(51-20-19-50-6)38(26-36(33)44-27-45-39)52-21-22-53-41(49)30-13-11-29(12-14-30)40(48)47-32-15-16-34-35(24-32)43(4,5)18-17-42(34,2)3/h1,8-16,23-27H,17-22H2,2-6H3,(H,47,48)(H,44,45,46) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of Ovine COX-1 preincubated for 15 mins by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50499594

(CHEMBL3740105)Show SMILES CC1(C)CCC(C)(C)c2cc(NC(=O)c3ccc(cc3)C(=O)OCCOc3cc4ncnc(Nc5cccc(c5)C#C)c4cc3OCCOC(=O)c3ccc(cc3)C(=O)Nc3ccc4c(c3)C(C)(C)CCC4(C)C)ccc12 Show InChI InChI=1S/C64H65N5O8/c1-10-40-12-11-13-45(34-40)67-56-48-37-54(74-30-32-76-59(72)43-18-14-41(15-19-43)57(70)68-46-22-24-49-51(35-46)63(6,7)28-26-61(49,2)3)55(38-53(48)65-39-66-56)75-31-33-77-60(73)44-20-16-42(17-21-44)58(71)69-47-23-25-50-52(36-47)64(8,9)29-27-62(50,4)5/h1,11-25,34-39H,26-33H2,2-9H3,(H,68,70)(H,69,71)(H,65,66,67) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX-2 preincubated for 15 mins by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM22360

(2-(acetyloxy)benzoate | 2-(acetyloxy)benzoic acid ...)Show InChI InChI=1S/C9H8O4/c1-6(10)13-8-5-3-2-4-7(8)9(11)12/h2-5H,1H3,(H,11,12) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) using tyrosine 4 as substrate by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of Ovine COX-1 preincubated for 15 mins by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM22360

(2-(acetyloxy)benzoate | 2-(acetyloxy)benzoic acid ...)Show InChI InChI=1S/C9H8O4/c1-6(10)13-8-5-3-2-4-7(8)9(11)12/h2-5H,1H3,(H,11,12) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of Ovine COX-1 preincubated for 15 mins by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50499594

(CHEMBL3740105)Show SMILES CC1(C)CCC(C)(C)c2cc(NC(=O)c3ccc(cc3)C(=O)OCCOc3cc4ncnc(Nc5cccc(c5)C#C)c4cc3OCCOC(=O)c3ccc(cc3)C(=O)Nc3ccc4c(c3)C(C)(C)CCC4(C)C)ccc12 Show InChI InChI=1S/C64H65N5O8/c1-10-40-12-11-13-45(34-40)67-56-48-37-54(74-30-32-76-59(72)43-18-14-41(15-19-43)57(70)68-46-22-24-49-51(35-46)63(6,7)28-26-61(49,2)3)55(38-53(48)65-39-66-56)75-31-33-77-60(73)44-20-16-42(17-21-44)58(71)69-47-23-25-50-52(36-47)64(8,9)29-27-62(50,4)5/h1,11-25,34-39H,26-33H2,2-9H3,(H,68,70)(H,69,71)(H,65,66,67) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of Ovine COX-1 preincubated for 15 mins by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50499593

(CHEMBL3739528)Show SMILES CC(C)Cc1ccc(cc1)C(C)C(=O)OCCOc1cc2ncnc(Nc3cccc(c3)C#C)c2cc1OCCOC(=O)C(C)c1ccc(CC(C)C)cc1 Show InChI InChI=1S/C46H51N3O6/c1-8-34-10-9-11-39(26-34)49-44-40-27-42(52-20-22-54-45(50)32(6)37-16-12-35(13-17-37)24-30(2)3)43(28-41(40)47-29-48-44)53-21-23-55-46(51)33(7)38-18-14-36(15-19-38)25-31(4)5/h1,9-19,26-33H,20-25H2,2-7H3,(H,47,48,49) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX-2 preincubated for 15 mins by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50499589

(CHEMBL3740595)Show SMILES COCCOc1cc2c(Nc3cccc(c3)C#C)ncnc2cc1OCCOC(=O)Cc1c(C)n(C(=O)c2ccc(Cl)cc2)c2ccc(OC)cc12 Show InChI InChI=1S/C40H35ClN4O7/c1-5-26-7-6-8-29(19-26)44-39-33-21-36(50-16-15-48-3)37(23-34(33)42-24-43-39)51-17-18-52-38(46)22-31-25(2)45(35-14-13-30(49-4)20-32(31)35)40(47)27-9-11-28(41)12-10-27/h1,6-14,19-21,23-24H,15-18,22H2,2-4H3,(H,42,43,44) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of Ovine COX-1 preincubated for 15 mins by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50103504

(CHEBI:9352 | Clinoril | Sulindac)Show SMILES C[S+]([O-])c1ccc(\C=C2\C(C)=C(CC(O)=O)c3cc(F)ccc23)cc1 |t:10| Show InChI InChI=1S/C20H17FO3S/c1-12-17(9-13-3-6-15(7-4-13)25(2)24)16-8-5-14(21)10-19(16)18(12)11-20(22)23/h3-10H,11H2,1-2H3,(H,22,23)/b17-9- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of Ovine COX-1 preincubated for 15 mins by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50499585

(CHEMBL3740850)Show SMILES COCCOc1cc2c(Nc3cccc(c3)C#C)ncnc2cc1OCCOC(=O)C(C)c1ccc2cc(OC)ccc2c1 Show InChI InChI=1S/C35H33N3O6/c1-5-24-7-6-8-28(17-24)38-34-30-20-32(42-14-13-40-3)33(21-31(30)36-22-37-34)43-15-16-44-35(39)23(2)25-9-10-27-19-29(41-4)12-11-26(27)18-25/h1,6-12,17-23H,13-16H2,2-4H3,(H,36,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 in human HCC827 cells assessed as decrease in PGE2 level by western blot analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50499585

(CHEMBL3740850)Show SMILES COCCOc1cc2c(Nc3cccc(c3)C#C)ncnc2cc1OCCOC(=O)C(C)c1ccc2cc(OC)ccc2c1 Show InChI InChI=1S/C35H33N3O6/c1-5-24-7-6-8-28(17-24)38-34-30-20-32(42-14-13-40-3)33(21-31(30)36-22-37-34)43-15-16-44-35(39)23(2)25-9-10-27-19-29(41-4)12-11-26(27)18-25/h1,6-12,17-23H,13-16H2,2-4H3,(H,36,37,38) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of Ovine COX-1 preincubated for 15 mins by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50499584

(CHEMBL3739771)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(=O)OCCOc3cc4ncnc(Nc5cccc(c5)C#C)c4cc3OCCOC(=O)Cc3c(C)n(C(=O)c4ccc(Cl)cc4)c4ccc(OC)cc34)c2c1 Show InChI InChI=1S/C58H47Cl2N5O10/c1-6-36-8-7-9-41(26-36)63-56-48-29-52(72-22-24-74-54(66)30-44-34(2)64(50-20-18-42(70-4)27-46(44)50)57(68)37-10-14-39(59)15-11-37)53(32-49(48)61-33-62-56)73-23-25-75-55(67)31-45-35(3)65(51-21-19-43(71-5)28-47(45)51)58(69)38-12-16-40(60)17-13-38/h1,7-21,26-29,32-33H,22-25,30-31H2,2-5H3,(H,61,62,63) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of Ovine COX-1 preincubated for 15 mins by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50499588

(CHEMBL3741399)Show SMILES CC(=O)Oc1ccccc1C(=O)OCCOc1cc2ncnc(Nc3cccc(c3)C#C)c2cc1OCCOC(=O)c1ccccc1OC(C)=O Show InChI InChI=1S/C38H31N3O10/c1-4-26-10-9-11-27(20-26)41-36-30-21-34(46-16-18-48-37(44)28-12-5-7-14-32(28)50-24(2)42)35(22-31(30)39-23-40-36)47-17-19-49-38(45)29-13-6-8-15-33(29)51-25(3)43/h1,5-15,20-23H,16-19H2,2-3H3,(H,39,40,41) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of Ovine COX-1 preincubated for 15 mins by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50499595

(CHEMBL3741026)Show SMILES COCCOc1cc2c(Nc3cccc(c3)C#C)ncnc2cc1OCCOC(=O)C(C)c1ccc(CC(C)C)cc1 Show InChI InChI=1S/C34H37N3O5/c1-6-25-8-7-9-28(19-25)37-33-29-20-31(40-15-14-39-5)32(21-30(29)35-22-36-33)41-16-17-42-34(38)24(4)27-12-10-26(11-13-27)18-23(2)3/h1,7-13,19-24H,14-18H2,2-5H3,(H,35,36,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of Ovine COX-1 preincubated for 15 mins by fluorescence analysis |
ACS Med Chem Lett 6: 1086-90 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00286
BindingDB Entry DOI: 10.7270/Q2ZG6W79 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data