

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378462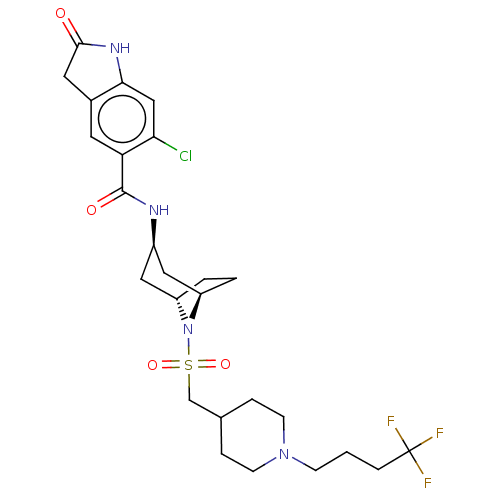 (6-chloro-2-oxo-N-((1R,3r,5S)-8-(((1-(4,4,4-trifluo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Non-competitive inhibition of full length N-terminal His-tagged SMYD3 (1 to 428 residues) (unknown origin) expressed in Escherichia coli using varyin... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378462 (6-chloro-2-oxo-N-((1R,3r,5S)-8-(((1-(4,4,4-trifluo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Non-competitive inhibition of full length N-terminal His-tagged SMYD3 (1 to 428 residues) (unknown origin) expressed in Escherichia coli using fixed ... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378459 (N-((1R,3r,5S)-8-((4-(benzylamino)piperidin-1-yl)su...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | PDB Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Non-competitive inhibition of full length N-terminal His-tagged SMYD3 (1 to 428 residues) (unknown origin) expressed in Escherichia coli using varyin... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378459 (N-((1R,3r,5S)-8-((4-(benzylamino)piperidin-1-yl)su...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | PDB Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Mixed type inhibition of full length N-terminal His-tagged SMYD3 (1 to 428 residues) (unknown origin) expressed in Escherichia coli using fixed N-ter... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378446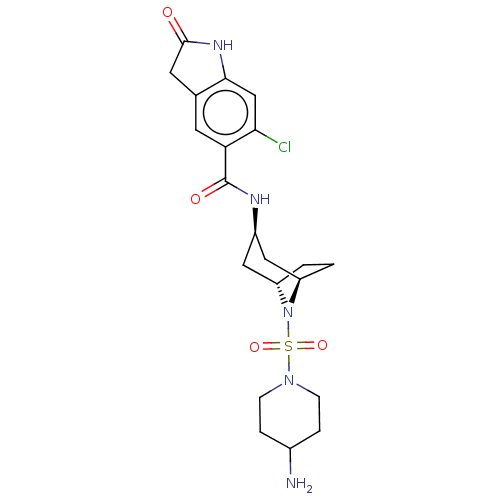 (N-((1R,3r,5S)-8-((4-aminopiperidin-1-yl)sulfonyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of full length N-terminal His-tagged SMYD3 (1 to 428 residues) (unknown origin) expressed in Escherichia coli using N-terminal GST-tagged ... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378444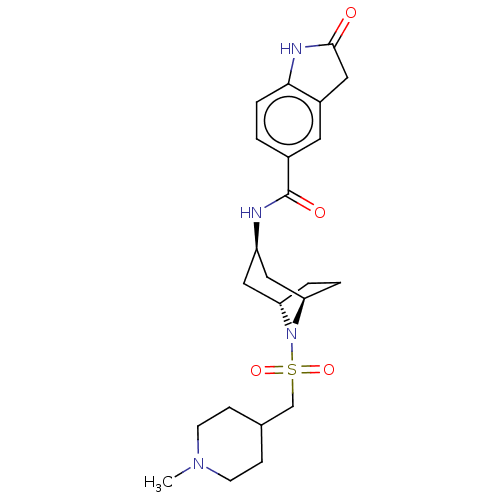 (N-((1R,3r,5S)-8-(((1-methylpiperidin-4-yl)methyl)s...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of full length N-terminal His-tagged SMYD3 (1 to 428 residues) (unknown origin) expressed in Escherichia coli using N-terminal GST-tagged ... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378445 (N-((1R,3r,5S)-8-((4-aminopiperidin-1-yl)sulfonyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of full length N-terminal His-tagged SMYD3 (1 to 428 residues) (unknown origin) expressed in Escherichia coli using N-terminal GST-tagged ... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378452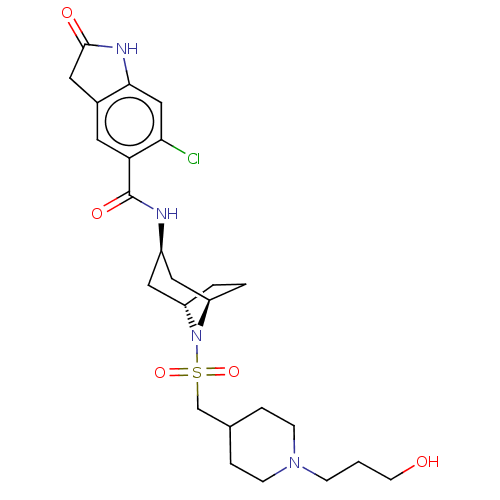 (6-chloro-N-((1R,3r,5S)-8-(((1-(3-hydroxypropyl)pip...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of full length N-terminal His-tagged SMYD3 (1 to 428 residues) (unknown origin) expressed in Escherichia coli using N-terminal GST-tagged ... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378453 (6-chloro-N-((1R,3r,5S)-8-((4-(methylamino)piperidi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of full length N-terminal His-tagged SMYD3 (1 to 428 residues) (unknown origin) expressed in Escherichia coli using N-terminal GST-tagged ... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378462 (6-chloro-2-oxo-N-((1R,3r,5S)-8-(((1-(4,4,4-trifluo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of full length N-terminal His-tagged SMYD3 (1 to 428 residues) (unknown origin) expressed in Escherichia coli using N-terminal GST-tagged ... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378461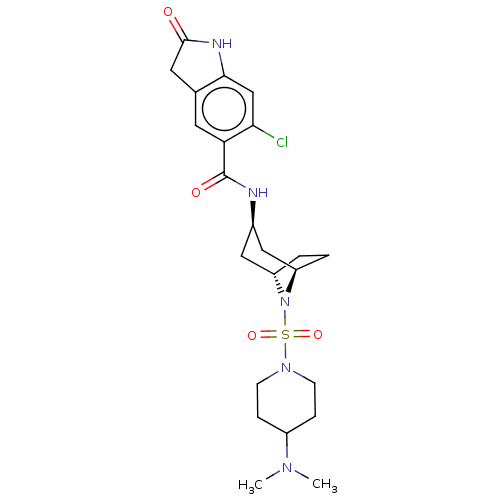 (6-chloro-N-((1R,3r,5S)-8-((4-(dimethylamino)piperi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of full length N-terminal His-tagged SMYD3 (1 to 428 residues) (unknown origin) expressed in Escherichia coli using N-terminal GST-tagged ... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378442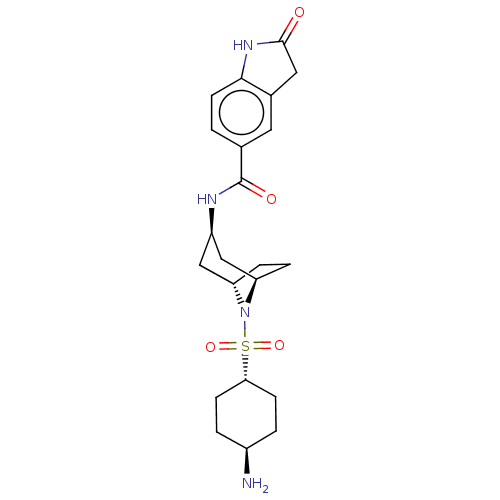 (N-((1R,3R,5S)-8-(((1r,4R)-4-aminocyclohexyl)sulfon...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of full length N-terminal His-tagged SMYD3 (1 to 428 residues) (unknown origin) expressed in Escherichia coli using N-terminal GST-tagged ... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50500875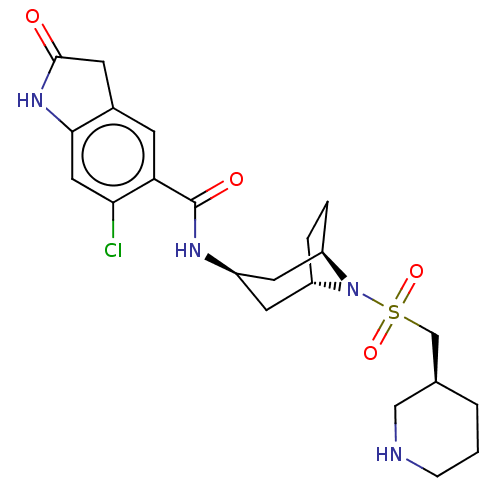 (CHEMBL3798745) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of full length N-terminal His-tagged SMYD3 (1 to 428 residues) (unknown origin) expressed in Escherichia coli using N-terminal GST-tagged ... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378464 (2-oxo-N-((1R,3r,5S)-8-((piperidin-4-ylmethyl)sulfo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of full length N-terminal His-tagged SMYD3 (1 to 428 residues) (unknown origin) expressed in Escherichia coli using N-terminal GST-tagged ... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378459 (N-((1R,3r,5S)-8-((4-(benzylamino)piperidin-1-yl)su...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of full length N-terminal His-tagged SMYD3 (1 to 428 residues) (unknown origin) expressed in Escherichia coli using N-terminal GST-tagged ... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378463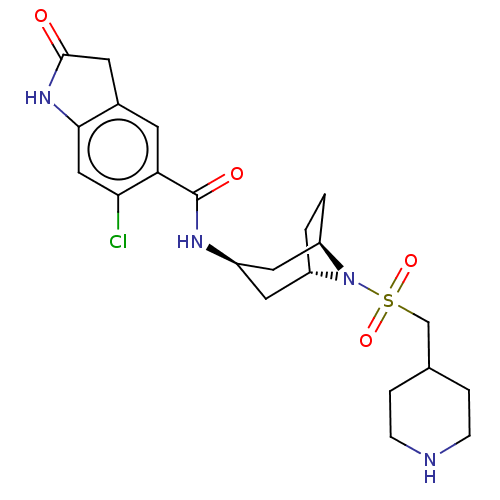 (6-chloro-2-oxo-N-((1R,3r,5S)-8-((piperidin-4-ylmet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of full length N-terminal His-tagged SMYD3 (1 to 428 residues) (unknown origin) expressed in Escherichia coli using N-terminal GST-tagged ... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378443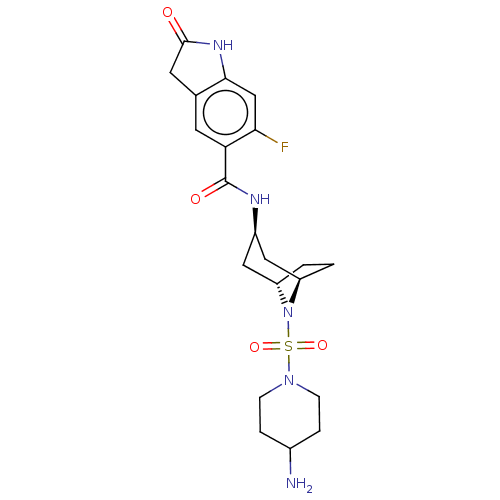 (N-((1R,3r,5S)-8-((4-aminopiperidin-1-yl)sulfonyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of full length N-terminal His-tagged SMYD3 (1 to 428 residues) (unknown origin) expressed in Escherichia coli using N-terminal GST-tagged ... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50500876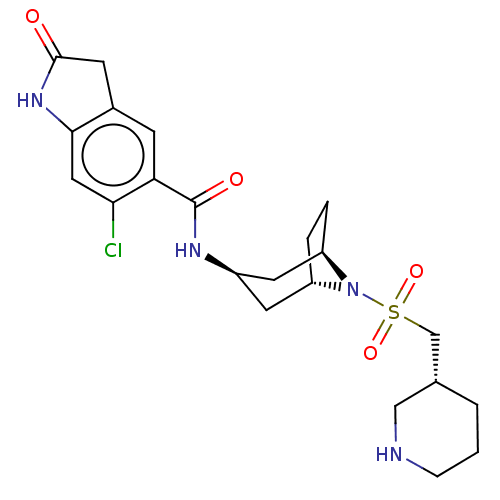 (CHEMBL3797575) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of full length N-terminal His-tagged SMYD3 (1 to 428 residues) (unknown origin) expressed in Escherichia coli using N-terminal GST-tagged ... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378473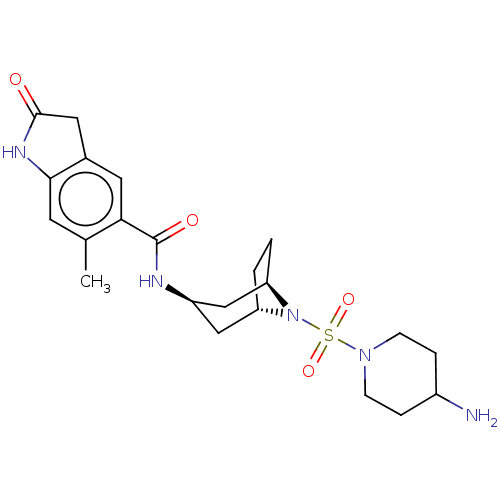 (N-((1R,3r,5S)-8-((4-aminopiperidin-1-yl)sulfonyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of full length N-terminal His-tagged SMYD3 (1 to 428 residues) (unknown origin) expressed in Escherichia coli using N-terminal GST-tagged ... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378478 (1-methyl-2-oxo-N-((1R,3r,5S)-8-((piperidin-4-ylmet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of full length N-terminal His-tagged SMYD3 (1 to 428 residues) (unknown origin) expressed in Escherichia coli using N-terminal GST-tagged ... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378462 (6-chloro-2-oxo-N-((1R,3r,5S)-8-(((1-(4,4,4-trifluo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of human SMYD3 expressed in HEK293T/17 cells using FLAG-tagged MEKK2 as substrate incubated for 30 mins in low air flow area followed by i... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378459 (N-((1R,3r,5S)-8-((4-(benzylamino)piperidin-1-yl)su...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of human SMYD3 expressed in HEK293T/17 cells using FLAG-tagged MEKK2 as substrate incubated for 30 mins in low air flow area followed by i... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378487 (2-oxo-N-(1-((piperidin-4-ylmethyl)sulfonyl)piperid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of full length N-terminal His-tagged SMYD3 (1 to 428 residues) (unknown origin) expressed in Escherichia coli using N-terminal GST-tagged ... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378488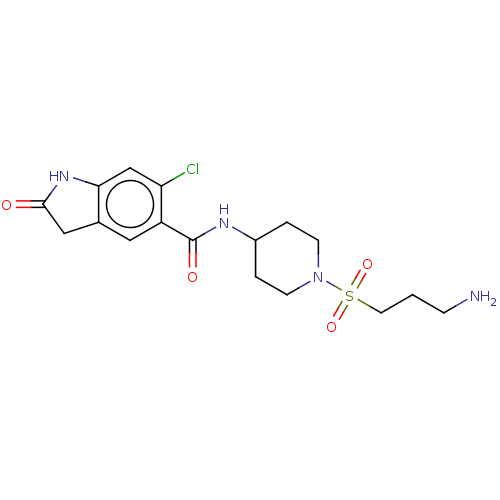 (N-(1-((3-aminopropyl)sulfonyl)piperidin-4-yl)-6-ch...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of full length N-terminal His-tagged SMYD3 (1 to 428 residues) (unknown origin) expressed in Escherichia coli using N-terminal GST-tagged ... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378491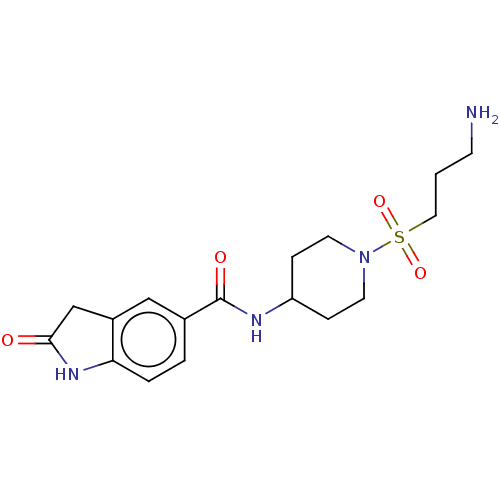 (N-(1-((3-aminopropyl)sulfonyl)piperidin-4-yl)-2-ox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of full length N-terminal His-tagged SMYD3 (1 to 428 residues) (unknown origin) expressed in Escherichia coli using N-terminal GST-tagged ... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378461 (6-chloro-N-((1R,3r,5S)-8-((4-(dimethylamino)piperi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of human SMYD3 expressed in HEK293T/17 cells using FLAG-tagged MEKK2 as substrate incubated for 30 mins in low air flow area followed by i... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378473 (N-((1R,3r,5S)-8-((4-aminopiperidin-1-yl)sulfonyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of human SMYD3 expressed in HEK293T/17 cells using FLAG-tagged MEKK2 as substrate incubated for 30 mins in low air flow area followed by i... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378453 (6-chloro-N-((1R,3r,5S)-8-((4-(methylamino)piperidi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of human SMYD3 expressed in HEK293T/17 cells using FLAG-tagged MEKK2 as substrate incubated for 30 mins in low air flow area followed by i... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378444 (N-((1R,3r,5S)-8-(((1-methylpiperidin-4-yl)methyl)s...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of human SMYD3 expressed in HEK293T/17 cells using FLAG-tagged MEKK2 as substrate incubated for 30 mins in low air flow area followed by i... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378443 (N-((1R,3r,5S)-8-((4-aminopiperidin-1-yl)sulfonyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of human SMYD3 expressed in HEK293T/17 cells using FLAG-tagged MEKK2 as substrate incubated for 30 mins in low air flow area followed by i... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50500876 (CHEMBL3797575) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of human SMYD3 expressed in HEK293T/17 cells using FLAG-tagged MEKK2 as substrate incubated for 30 mins in low air flow area followed by i... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378452 (6-chloro-N-((1R,3r,5S)-8-(((1-(3-hydroxypropyl)pip...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of human SMYD3 expressed in HEK293T/17 cells using FLAG-tagged MEKK2 as substrate incubated for 30 mins in low air flow area followed by i... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378445 (N-((1R,3r,5S)-8-((4-aminopiperidin-1-yl)sulfonyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of human SMYD3 expressed in HEK293T/17 cells using FLAG-tagged MEKK2 as substrate incubated for 30 mins in low air flow area followed by i... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378446 (N-((1R,3r,5S)-8-((4-aminopiperidin-1-yl)sulfonyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of human SMYD3 expressed in HEK293T/17 cells using FLAG-tagged MEKK2 as substrate incubated for 30 mins in low air flow area followed by i... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50500875 (CHEMBL3798745) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of human SMYD3 expressed in HEK293T/17 cells using FLAG-tagged MEKK2 as substrate incubated for 30 mins in low air flow area followed by i... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378442 (N-((1R,3R,5S)-8-(((1r,4R)-4-aminocyclohexyl)sulfon...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of human SMYD3 expressed in HEK293T/17 cells using FLAG-tagged MEKK2 as substrate incubated for 30 mins in low air flow area followed by i... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378463 (6-chloro-2-oxo-N-((1R,3r,5S)-8-((piperidin-4-ylmet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of human SMYD3 expressed in HEK293T/17 cells using FLAG-tagged MEKK2 as substrate incubated for 30 mins in low air flow area followed by i... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378464 (2-oxo-N-((1R,3r,5S)-8-((piperidin-4-ylmethyl)sulfo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of human SMYD3 expressed in HEK293T/17 cells using FLAG-tagged MEKK2 as substrate incubated for 30 mins in low air flow area followed by i... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378478 (1-methyl-2-oxo-N-((1R,3r,5S)-8-((piperidin-4-ylmet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of human SMYD3 expressed in HEK293T/17 cells using FLAG-tagged MEKK2 as substrate incubated for 30 mins in low air flow area followed by i... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378488 (N-(1-((3-aminopropyl)sulfonyl)piperidin-4-yl)-6-ch...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of human SMYD3 expressed in HEK293T/17 cells using FLAG-tagged MEKK2 as substrate incubated for 30 mins in low air flow area followed by i... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378491 (N-(1-((3-aminopropyl)sulfonyl)piperidin-4-yl)-2-ox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of human SMYD3 expressed in HEK293T/17 cells using FLAG-tagged MEKK2 as substrate incubated for 30 mins in low air flow area followed by i... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM377919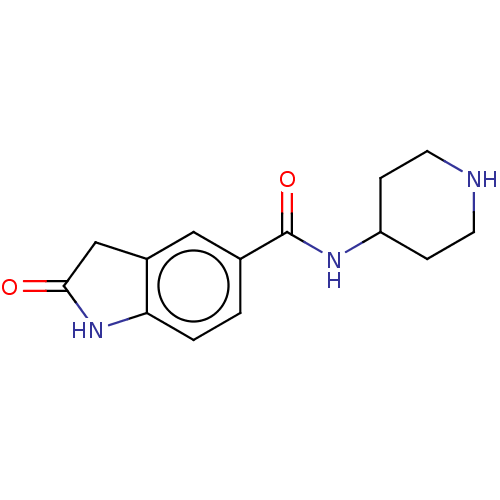 (2-oxo-N-(piperidin-4-yl)indoline-5-carboxamide | U...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of human SMYD3 expressed in HEK293T/17 cells using FLAG-tagged MEKK2 as substrate incubated for 30 mins in low air flow area followed by i... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378487 (2-oxo-N-(1-((piperidin-4-ylmethyl)sulfonyl)piperid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of human SMYD3 expressed in HEK293T/17 cells using FLAG-tagged MEKK2 as substrate incubated for 30 mins in low air flow area followed by i... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM377919 (2-oxo-N-(piperidin-4-yl)indoline-5-carboxamide | U...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of full length N-terminal His-tagged SMYD3 (1 to 428 residues) (unknown origin) expressed in Escherichia coli using N-terminal GST-tagged ... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM378462 (6-chloro-2-oxo-N-((1R,3r,5S)-8-(((1-(4,4,4-trifluo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of SMYD2 (unknown origin) | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM378459 (N-((1R,3r,5S)-8-((4-(benzylamino)piperidin-1-yl)su...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of SMYD2 (unknown origin) | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||