

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50017721 (1-Methyl-4-(5H-dibenzo(a,d)cycloheptenylidene)pipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University (TMDU) Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from Sprague-Dawley rat cerebral cortex 5-HT2A receptor after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 24: 4318-4323 (2016) Article DOI: 10.1016/j.bmc.2016.07.024 BindingDB Entry DOI: 10.7270/Q2959KGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50097224 (1-Methyl-4-thioxanthen-9-ylidene-piperidine | 1-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University (TMDU) Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from Sprague-Dawley rat cerebral cortex 5-HT2A receptor after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 24: 4318-4323 (2016) Article DOI: 10.1016/j.bmc.2016.07.024 BindingDB Entry DOI: 10.7270/Q2959KGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50097222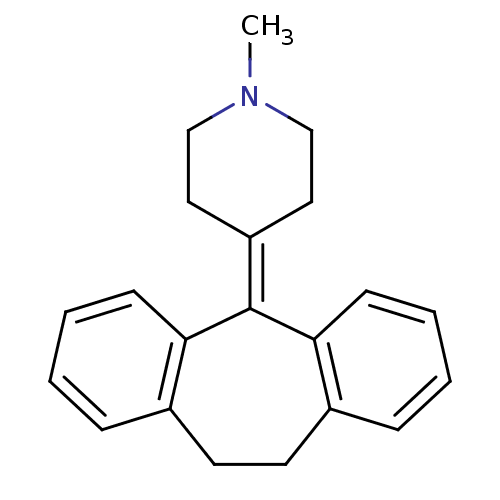 (4-(10,11-Dihydro-dibenzo[a,d]cyclohepten-5-ylidene...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University (TMDU) Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from Sprague-Dawley rat cerebral cortex 5-HT2A receptor after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 24: 4318-4323 (2016) Article DOI: 10.1016/j.bmc.2016.07.024 BindingDB Entry DOI: 10.7270/Q2959KGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50097215 (4-Fluoren-9-ylidene-1-methyl-piperidine | CHEMBL34...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 199 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University (TMDU) Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from Sprague-Dawley rat cerebral cortex 5-HT2A receptor after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 24: 4318-4323 (2016) Article DOI: 10.1016/j.bmc.2016.07.024 BindingDB Entry DOI: 10.7270/Q2959KGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SETD7 (Homo sapiens (Human)) | BDBM50017721 (1-Methyl-4-(5H-dibenzo(a,d)cycloheptenylidene)pipe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University (TMDU) Curated by ChEMBL | Assay Description Inhibition of recombinant SET7/9 (unknown origin) using biotinylated histone H3-derived peptide/SAM as substrate after 60 mins by AlphaLISA assay | Bioorg Med Chem 24: 4318-4323 (2016) Article DOI: 10.1016/j.bmc.2016.07.024 BindingDB Entry DOI: 10.7270/Q2959KGV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase SETD7 (Homo sapiens (Human)) | BDBM50097224 (1-Methyl-4-thioxanthen-9-ylidene-piperidine | 1-me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University (TMDU) Curated by ChEMBL | Assay Description Inhibition of recombinant SET7/9 (unknown origin) using biotinylated histone H3-derived peptide/SAM as substrate after 60 mins by AlphaLISA assay | Bioorg Med Chem 24: 4318-4323 (2016) Article DOI: 10.1016/j.bmc.2016.07.024 BindingDB Entry DOI: 10.7270/Q2959KGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SETD7 (Homo sapiens (Human)) | BDBM50097216 (1-Methyl-4-xanthen-9-ylidene-piperidine | CHEMBL15...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University (TMDU) Curated by ChEMBL | Assay Description Inhibition of recombinant SET7/9 (unknown origin) using biotinylated histone H3-derived peptide/SAM as substrate after 60 mins by AlphaLISA assay | Bioorg Med Chem 24: 4318-4323 (2016) Article DOI: 10.1016/j.bmc.2016.07.024 BindingDB Entry DOI: 10.7270/Q2959KGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SETD7 (Homo sapiens (Human)) | BDBM50097215 (4-Fluoren-9-ylidene-1-methyl-piperidine | CHEMBL34...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University (TMDU) Curated by ChEMBL | Assay Description Inhibition of recombinant SET7/9 (unknown origin) using biotinylated histone H3-derived peptide/SAM as substrate after 60 mins by AlphaLISA assay | Bioorg Med Chem 24: 4318-4323 (2016) Article DOI: 10.1016/j.bmc.2016.07.024 BindingDB Entry DOI: 10.7270/Q2959KGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SETD7 (Homo sapiens (Human)) | BDBM50097222 (4-(10,11-Dihydro-dibenzo[a,d]cyclohepten-5-ylidene...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.91E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University (TMDU) Curated by ChEMBL | Assay Description Inhibition of recombinant SET7/9 (unknown origin) using biotinylated histone H3-derived peptide/SAM as substrate after 60 mins by AlphaLISA assay | Bioorg Med Chem 24: 4318-4323 (2016) Article DOI: 10.1016/j.bmc.2016.07.024 BindingDB Entry DOI: 10.7270/Q2959KGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SETD7 (Homo sapiens (Human)) | BDBM50097226 (4-Benzhydrylidene-1-methyl-piperidine | CHEMBL3475...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University (TMDU) Curated by ChEMBL | Assay Description Inhibition of recombinant SET7/9 (unknown origin) using biotinylated histone H3-derived peptide/SAM as substrate after 60 mins by AlphaLISA assay | Bioorg Med Chem 24: 4318-4323 (2016) Article DOI: 10.1016/j.bmc.2016.07.024 BindingDB Entry DOI: 10.7270/Q2959KGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SETD7 (Homo sapiens (Human)) | BDBM50187211 (CHEMBL20468) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University (TMDU) Curated by ChEMBL | Assay Description Inhibition of recombinant SET7/9 (unknown origin) using biotinylated histone H3-derived peptide/SAM as substrate after 60 mins by AlphaLISA assay | Bioorg Med Chem 24: 4318-4323 (2016) Article DOI: 10.1016/j.bmc.2016.07.024 BindingDB Entry DOI: 10.7270/Q2959KGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SETD7 (Homo sapiens (Human)) | BDBM50187210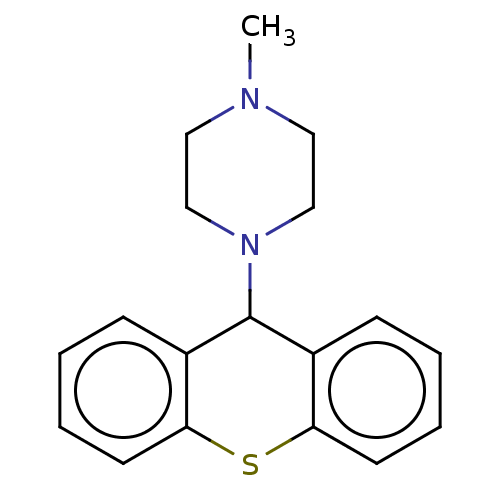 (CHEMBL3827652) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University (TMDU) Curated by ChEMBL | Assay Description Inhibition of recombinant SET7/9 (unknown origin) using biotinylated histone H3-derived peptide/SAM as substrate after 60 mins by AlphaLISA assay | Bioorg Med Chem 24: 4318-4323 (2016) Article DOI: 10.1016/j.bmc.2016.07.024 BindingDB Entry DOI: 10.7270/Q2959KGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SETD7 (Homo sapiens (Human)) | BDBM50187209 (CHEMBL3828123) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University (TMDU) Curated by ChEMBL | Assay Description Inhibition of recombinant SET7/9 (unknown origin) using biotinylated histone H3-derived peptide/SAM as substrate after 60 mins by AlphaLISA assay | Bioorg Med Chem 24: 4318-4323 (2016) Article DOI: 10.1016/j.bmc.2016.07.024 BindingDB Entry DOI: 10.7270/Q2959KGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SETD7 (Homo sapiens (Human)) | BDBM50187208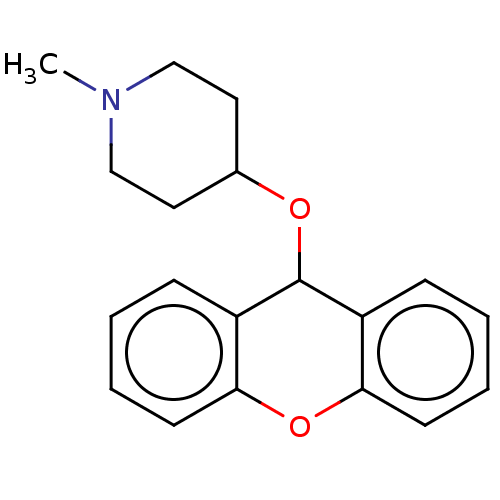 (CHEMBL3827386) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University (TMDU) Curated by ChEMBL | Assay Description Inhibition of recombinant SET7/9 (unknown origin) using biotinylated histone H3-derived peptide/SAM as substrate after 60 mins by AlphaLISA assay | Bioorg Med Chem 24: 4318-4323 (2016) Article DOI: 10.1016/j.bmc.2016.07.024 BindingDB Entry DOI: 10.7270/Q2959KGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SETD7 (Homo sapiens (Human)) | BDBM50187207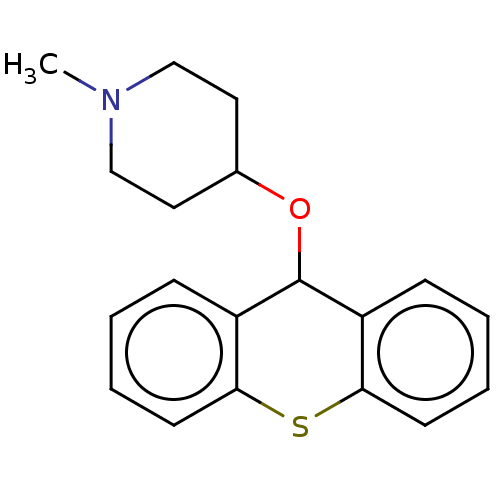 (CHEMBL3827431) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University (TMDU) Curated by ChEMBL | Assay Description Inhibition of recombinant SET7/9 (unknown origin) using biotinylated histone H3-derived peptide/SAM as substrate after 60 mins by AlphaLISA assay | Bioorg Med Chem 24: 4318-4323 (2016) Article DOI: 10.1016/j.bmc.2016.07.024 BindingDB Entry DOI: 10.7270/Q2959KGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SETD7 (Homo sapiens (Human)) | BDBM50187212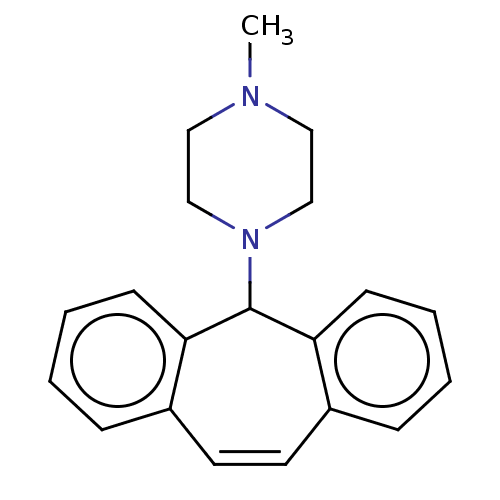 (CHEMBL3828393) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University (TMDU) Curated by ChEMBL | Assay Description Inhibition of recombinant SET7/9 (unknown origin) using biotinylated histone H3-derived peptide/SAM as substrate after 60 mins by AlphaLISA assay | Bioorg Med Chem 24: 4318-4323 (2016) Article DOI: 10.1016/j.bmc.2016.07.024 BindingDB Entry DOI: 10.7270/Q2959KGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||