

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50239038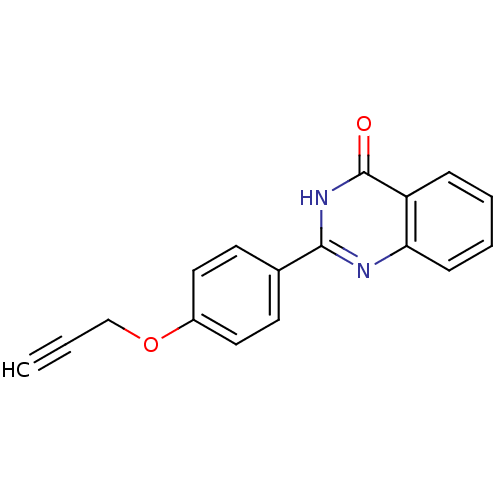 (CHEMBL4074105) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP1B1 expressed in yeast microsomal membranes using 7-ethoxyresorufin as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50188337 (CHEMBL2058057) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP1B1 expressed in yeast microsomal membranes using 7-ethoxyresorufin as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50239037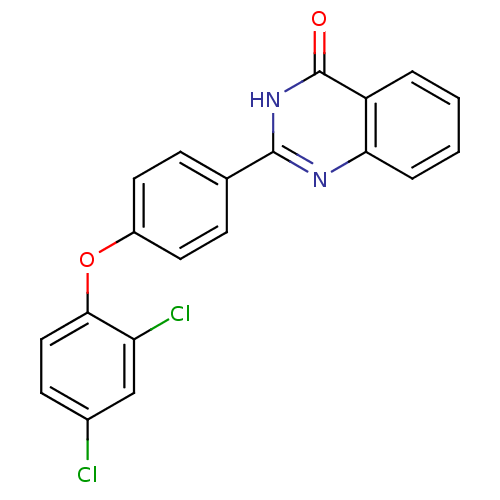 (CHEMBL4100866) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP1B1 expressed in yeast microsomal membranes using 7-ethoxyresorufin as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50014323 (2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP1B1 expressed in Escherichia coli DH5[alpha] using 7-ethoxyresorufin/3-cyano-7-ethoxycoumarin as substrate in pres... | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50014323 (2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP1A1 expressed in yeast microsomal membranes using 7-ethoxyresorufin/3-cyano-7-ethoxycoumarin as substrate by fluorescence assa... | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50188337 (CHEMBL2058057) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human liver CYP1B1 expressed in HEK293 cells using 7-ethoxyresorufin as substrate pretreated for 30 mins followed by substr... | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50014323 (2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP1A2 expressed in yeast microsomal membranes using 7-ethoxyresorufin/3-cyano-7-ethoxycoumarin/7-ethoxy-methyloxy-3-cyanocoumari... | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50239035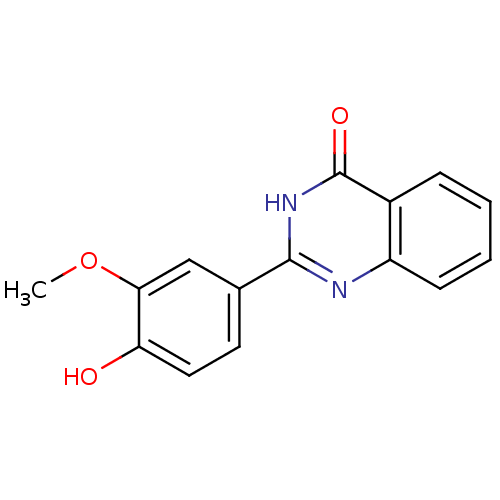 (CHEMBL4081914) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human liver CYP1B1 expressed in HEK293 cells using 7-ethoxyresorufin as substrate pretreated for 30 mins followed by substr... | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50239038 (CHEMBL4074105) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human liver CYP1B1 expressed in HEK293 cells using 7-ethoxyresorufin as substrate pretreated for 30 mins followed by substr... | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50014323 (2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP1B1 expressed in yeast microsomal membranes using 7-ethoxyresorufin as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50019170 (2-(3'-Hydroxy-4'-methoxyphenyl)quinazolin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP1B1 expressed in yeast microsomal membranes using 7-ethoxyresorufin as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50239039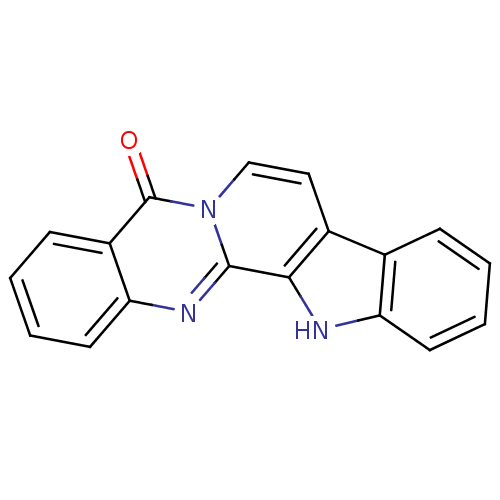 (CHEMBL4098122) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of CYP1B1 (unknown origin) | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50188337 (CHEMBL2058057) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP1B1 expressed in yeast cells using 7-ethoxyresorufin as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50014323 (2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP1A1 expressed in Escherichia coli DH5[alpha] using 7-ethoxyresorufin/3-cyano-7-ethoxycoumarin as substrate in pres... | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50239037 (CHEMBL4100866) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP1B1 expressed in yeast cells using 7-ethoxyresorufin as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50239038 (CHEMBL4074105) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP1B1 expressed in yeast cells using 7-ethoxyresorufin as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM7460 (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of CYP1B1 (unknown origin) | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50019170 (2-(3'-Hydroxy-4'-methoxyphenyl)quinazolin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human liver CYP1B1 expressed in HEK293 cells using 7-ethoxyresorufin as substrate pretreated for 30 mins followed by substr... | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50239034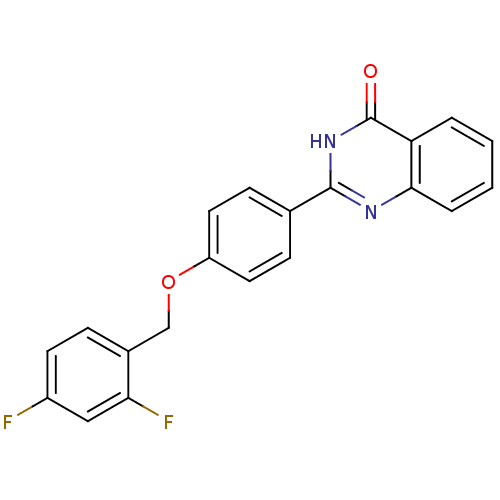 (CHEMBL4084413) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human liver CYP1B1 expressed in HEK293 cells using 7-ethoxyresorufin as substrate pretreated for 30 mins followed by substr... | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50239034 (CHEMBL4084413) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP1B1 expressed in yeast cells using 7-ethoxyresorufin as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50239035 (CHEMBL4081914) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 141 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP1B1 expressed in yeast cells using 7-ethoxyresorufin as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50239037 (CHEMBL4100866) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 149 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human liver CYP1B1 expressed in HEK293 cells using 7-ethoxyresorufin as substrate pretreated for 30 mins followed by substr... | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50019170 (2-(3'-Hydroxy-4'-methoxyphenyl)quinazolin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 151 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP1B1 expressed in yeast cells using 7-ethoxyresorufin as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50239039 (CHEMBL4098122) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of CYP1A1 (unknown origin) | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50239034 (CHEMBL4084413) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 268 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP1B1 expressed in yeast microsomal membranes using 7-ethoxyresorufin as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50239034 (CHEMBL4084413) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 477 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP1A1 expressed in yeast microsomal membranes using 7-ethoxyresorufin/3-cyano-7-ethoxycoumarin as substrate by fluorescence assa... | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50239038 (CHEMBL4074105) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP1A1 expressed in yeast cells using 7-ethoxyresorufin/3-cyano-7-ethoxycoumarin as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50239037 (CHEMBL4100866) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human liver CYP1A1 expressed in HEK293 cells using 7-ethoxyresorufin as substrate pretreated for 30 mins followed by substr... | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50239037 (CHEMBL4100866) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP1A1 expressed in yeast cells using 7-ethoxyresorufin/3-cyano-7-ethoxycoumarin as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM7460 (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of CYP1A1 (unknown origin) | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50188337 (CHEMBL2058057) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human liver CYP1A1 expressed in HEK293 cells using 7-ethoxyresorufin as substrate pretreated for 30 mins followed by substr... | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50239035 (CHEMBL4081914) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human liver CYP1A1 expressed in HEK293 cells using 7-ethoxyresorufin as substrate pretreated for 30 mins followed by substr... | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50239038 (CHEMBL4074105) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human liver CYP1A1 expressed in HEK293 cells using 7-ethoxyresorufin as substrate pretreated for 30 mins followed by substr... | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50188337 (CHEMBL2058057) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP1A1 expressed in yeast cells using 7-ethoxyresorufin/3-cyano-7-ethoxycoumarin as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50239035 (CHEMBL4081914) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP1B1 expressed in yeast microsomal membranes using 7-ethoxyresorufin as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50019170 (2-(3'-Hydroxy-4'-methoxyphenyl)quinazolin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP1A1 expressed in yeast cells using 7-ethoxyresorufin/3-cyano-7-ethoxycoumarin as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50239034 (CHEMBL4084413) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP1A1 expressed in yeast cells using 7-ethoxyresorufin/3-cyano-7-ethoxycoumarin as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50239035 (CHEMBL4081914) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP1A1 expressed in yeast cells using 7-ethoxyresorufin/3-cyano-7-ethoxycoumarin as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50019170 (2-(3'-Hydroxy-4'-methoxyphenyl)quinazolin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP1A1 expressed in yeast microsomal membranes using 7-ethoxyresorufin/3-cyano-7-ethoxycoumarin as substrate by fluorescence assa... | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50019170 (2-(3'-Hydroxy-4'-methoxyphenyl)quinazolin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human liver CYP1A1 expressed in HEK293 cells using 7-ethoxyresorufin as substrate pretreated for 30 mins followed by substr... | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50239034 (CHEMBL4084413) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human liver CYP1A1 expressed in HEK293 cells using 7-ethoxyresorufin as substrate pretreated for 30 mins followed by substr... | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50239034 (CHEMBL4084413) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP1A2 expressed in yeast microsomal membranes using 7-ethoxyresorufin/3-cyano-7-ethoxycoumarin/7-ethoxy-methyloxy-3-cyanocoumari... | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50239035 (CHEMBL4081914) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP1A1 expressed in yeast microsomal membranes using 7-ethoxyresorufin/3-cyano-7-ethoxycoumarin as substrate by fluorescence assa... | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50188337 (CHEMBL2058057) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Ability to displace [3H]spiperone binding from anterior pituitary Dopamine receptor D2 in the presence of 100 uM GTP | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50014323 (2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 expressed in yeast microsomal membranes using 7-ethoxy-methyloxy-3-cyanocoumarin as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50239037 (CHEMBL4100866) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 expressed in yeast microsomal membranes using 7-ethoxy-methyloxy-3-cyanocoumarin as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50014323 (2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human liver CYP1B1 expressed in HEK293 cells using 7-ethoxyresorufin as substrate pretreated for 30 mins followed by substr... | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50019170 (2-(3'-Hydroxy-4'-methoxyphenyl)quinazolin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 expressed in yeast microsomal membranes using dibenzylfluorescein as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50014323 (2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human liver CYP1A1 expressed in HEK293 cells using 7-ethoxyresorufin as substrate pretreated for 30 mins followed by substr... | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50019170 (2-(3'-Hydroxy-4'-methoxyphenyl)quinazolin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP1A2 expressed in yeast microsomal membranes using 7-ethoxyresorufin/3-cyano-7-ethoxycoumarin/7-ethoxy-methyloxy-3-cyanocoumari... | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50239037 (CHEMBL4100866) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP1A2 expressed in yeast microsomal membranes using 7-ethoxyresorufin/3-cyano-7-ethoxycoumarin/7-ethoxy-methyloxy-3-cyanocoumari... | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50239035 (CHEMBL4081914) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP1A2 expressed in yeast microsomal membranes using 7-ethoxyresorufin/3-cyano-7-ethoxycoumarin/7-ethoxy-methyloxy-3-cyanocoumari... | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50239038 (CHEMBL4074105) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Ability to displace [3H]spiperone binding from anterior pituitary Dopamine receptor D2 in the absence of GTP | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50019170 (2-(3'-Hydroxy-4'-methoxyphenyl)quinazolin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 expressed in yeast microsomal membranes using 7-ethoxy-methyloxy-3-cyanocoumarin as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50239035 (CHEMBL4081914) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 expressed in yeast microsomal membranes using dibenzylfluorescein as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50239037 (CHEMBL4100866) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 expressed in yeast microsomal membranes using dibenzylfluorescein as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50239038 (CHEMBL4074105) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Ability to displace [3H]spiperone binding from anterior pituitary Dopamine receptor D2 in the absence of GTP | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50014323 (2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP2C9 expressed in yeast microsomal membranes using 3-cyano-7-ethoxycoumarin as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50014323 (2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP1B1 expressed in yeast cells using 7-ethoxyresorufin as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50188337 (CHEMBL2058057) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP1A2 expressed in yeast microsomal membranes using 7-ethoxyresorufin/3-cyano-7-ethoxycoumarin/7-ethoxy-methyloxy-3-cyanocoumari... | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50188337 (CHEMBL2058057) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 expressed in yeast microsomal membranes using 7-ethoxy-methyloxy-3-cyanocoumarin as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50188337 (CHEMBL2058057) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 expressed in yeast microsomal membranes using dibenzylfluorescein as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50239034 (CHEMBL4084413) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 expressed in yeast microsomal membranes using 7-ethoxy-methyloxy-3-cyanocoumarin as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50014323 (2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP2C19 expressed in yeast microsomal membranes using 3-cyano-7-ethoxycoumarin as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50239034 (CHEMBL4084413) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 expressed in yeast microsomal membranes using dibenzylfluorescein as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50014323 (2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 expressed in yeast microsomal membranes using dibenzylfluorescein as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50014323 (2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP1A1 expressed in yeast cells using 7-ethoxyresorufin/3-cyano-7-ethoxycoumarin as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50239038 (CHEMBL4074105) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 expressed in yeast microsomal membranes using dibenzylfluorescein as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50239035 (CHEMBL4081914) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 expressed in yeast microsomal membranes using 7-ethoxy-methyloxy-3-cyanocoumarin as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50239037 (CHEMBL4100866) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP1A1 expressed in yeast microsomal membranes using 7-ethoxyresorufin/3-cyano-7-ethoxycoumarin as substrate by fluorescence assa... | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50239037 (CHEMBL4100866) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP2C9 expressed in yeast microsomal membranes using 3-cyano-7-ethoxycoumarin as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50239034 (CHEMBL4084413) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP2C19 expressed in yeast microsomal membranes using 3-cyano-7-ethoxycoumarin as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50239034 (CHEMBL4084413) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP2C9 expressed in yeast microsomal membranes using 3-cyano-7-ethoxycoumarin as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50019170 (2-(3'-Hydroxy-4'-methoxyphenyl)quinazolin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP2C19 expressed in yeast microsomal membranes using 3-cyano-7-ethoxycoumarin as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50188337 (CHEMBL2058057) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP2C9 expressed in yeast microsomal membranes using 3-cyano-7-ethoxycoumarin as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50239038 (CHEMBL4074105) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP2C9 expressed in yeast microsomal membranes using 3-cyano-7-ethoxycoumarin as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50239035 (CHEMBL4081914) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP2C9 expressed in yeast microsomal membranes using 3-cyano-7-ethoxycoumarin as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50239038 (CHEMBL4074105) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP2C19 expressed in yeast microsomal membranes using 3-cyano-7-ethoxycoumarin as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50239035 (CHEMBL4081914) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP2C19 expressed in yeast microsomal membranes using 3-cyano-7-ethoxycoumarin as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50188337 (CHEMBL2058057) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP2C19 expressed in yeast microsomal membranes using 3-cyano-7-ethoxycoumarin as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50019170 (2-(3'-Hydroxy-4'-methoxyphenyl)quinazolin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP2C9 expressed in yeast microsomal membranes using 3-cyano-7-ethoxycoumarin as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50239037 (CHEMBL4100866) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP2C19 expressed in yeast microsomal membranes using 3-cyano-7-ethoxycoumarin as substrate by fluorescence assay | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50239038 (CHEMBL4074105) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP1A1 expressed in yeast microsomal membranes using 7-ethoxyresorufin/3-cyano-7-ethoxycoumarin as substrate by fluorescence assa... | Eur J Med Chem 130: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.02.032 BindingDB Entry DOI: 10.7270/Q2JQ139G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||