

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mannan-binding lectin serine protease 2 (Homo sapiens) | BDBM50462098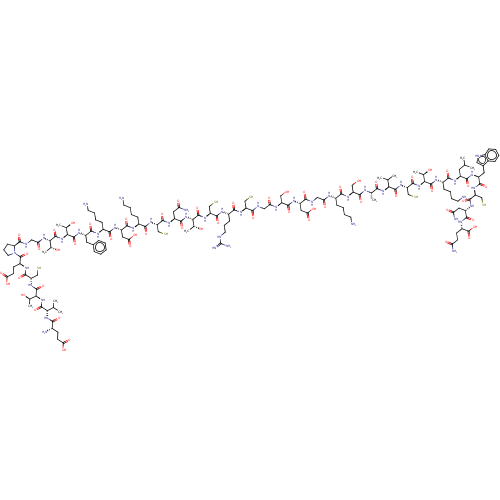 (CHEMBL4242955) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of recombinant CP1-CCP2-SP fragment of MASP-2 (unknown origin) | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1s subcomponent (Homo sapiens (Human)) | BDBM50462110 (CHEMBL4244141) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of C1s (unknown origin) using Cbz-Gly-Arg-S-Bzl as substrate preincubated with substrate for 15 mins followed by enzyme addition and measu... | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannan-binding lectin serine protease 1 (Homo sapiens) | BDBM50462088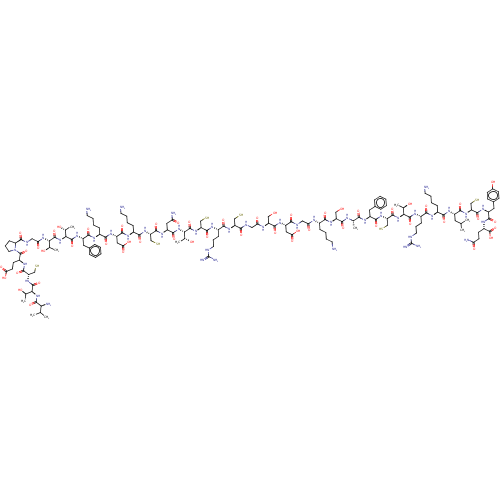 (CHEMBL4250967) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of recombinant CP1-CCP2-SP fragment of MASP-1 (unknown origin) | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1s subcomponent (Homo sapiens (Human)) | BDBM50462090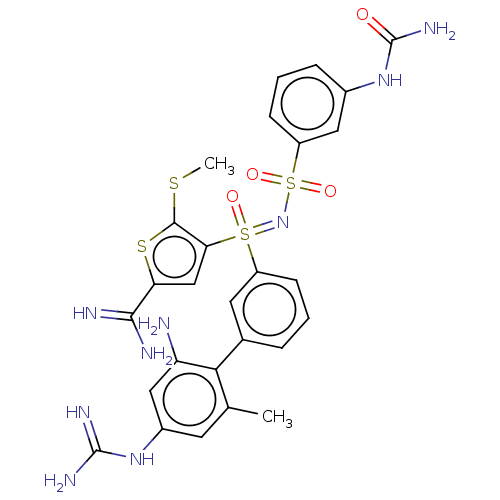 (CHEMBL4242095) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human plasma C1s using Cbz-Gly-Arg-S-Bzl as substrate preincubated with substrate for 15 mins followed by enzyme addition and measured ... | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C5a anaphylatoxin chemotactic receptor 1 (Homo sapiens (Human)) | BDBM50462094 (CHEMBL4250011) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Antagonist activity at recombinant human C5aR1 expressed in Sf9 insect cell membranes co-expressing G-alphai2,G-beta1 and G-gamma2 assessed as inhibi... | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1s subcomponent (Homo sapiens (Human)) | BDBM50462111 (CHEMBL4239924) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of C1s (unknown origin) using Cbz-Gly-Arg-S-Bzl as substrate preincubated with substrate for 15 mins followed by enzyme addition and measu... | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannan-binding lectin serine protease 1 (Homo sapiens) | BDBM50462102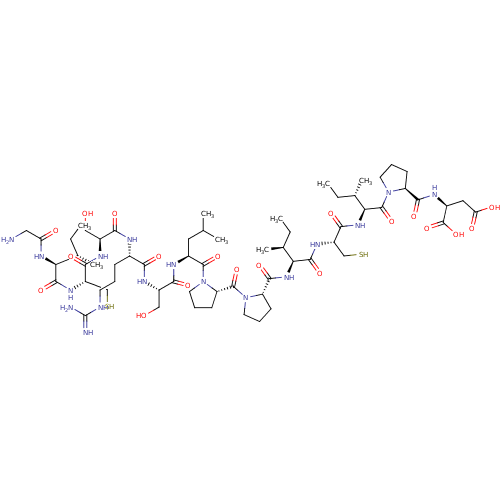 (CHEMBL4240381) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of MASP-1 (unknown origin) using Z-L-LysSBzl hydrochloride as substrate preincubated for 1 hr followed by substrate addition | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannan-binding lectin serine protease 2 (Homo sapiens) | BDBM50462099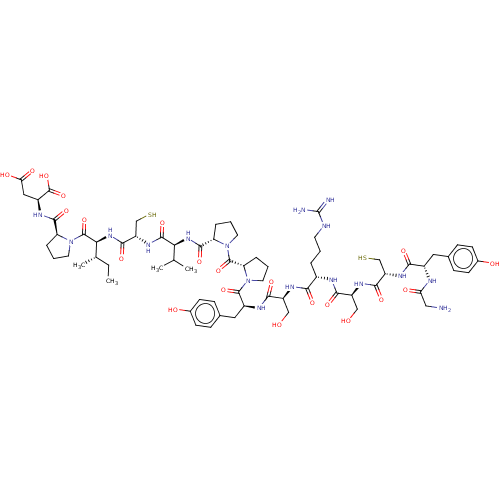 (CHEMBL4246684) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of MASP-2 (unknown origin) using Z-L-LysSBzl hydrochloride as substrate preincubated for 1 hr followed by substrate addition | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannan-binding lectin serine protease 2 (Homo sapiens) | BDBM50462090 (CHEMBL4242095) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human His-tagged MASP-2 (Cys300 to Phe686 residues) expressed in baculovirus infected insect cells using Z-Gly-Arg-S-Bzl as substrate p... | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannan-binding lectin serine protease 2 (Homo sapiens) | BDBM50462102 (CHEMBL4240381) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of MASP-2 (unknown origin) using Z-L-LysSBzl hydrochloride as substrate preincubated for 1 hr followed by substrate addition | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C5a anaphylatoxin chemotactic receptor 1 (Homo sapiens (Human)) | BDBM50462093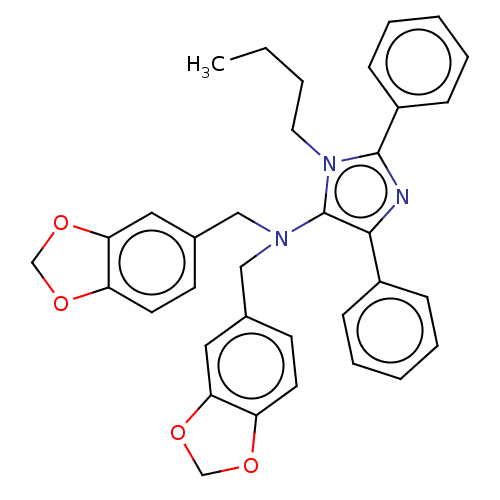 (CHEMBL4245250) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Antagonist activity at C5aR1 in human HL60 cells assessed as inhibition of recombinant human C5a-induced oxidative burst after 30 mins by fluorescenc... | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor B (Homo sapiens (Human)) | BDBM251408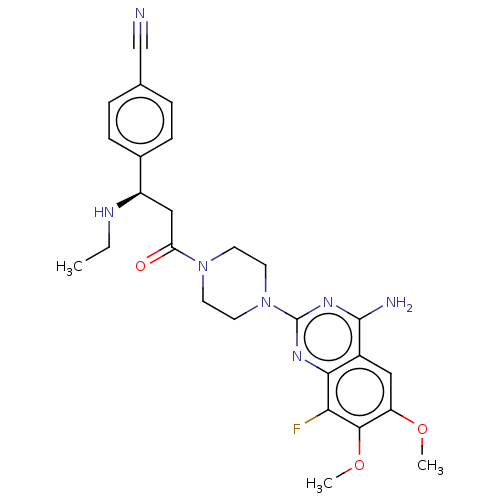 (US9452990, 90) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of recombinant human factor B expressed in drosophila cells assessed as decrease in C3a formation using CVF-Bb complex and human complemen... | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C5a anaphylatoxin chemotactic receptor 1 (Homo sapiens (Human)) | BDBM50462093 (CHEMBL4245250) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Antagonist activity at C5aR1 in human U937 cells assessed as inhibition of recombinant human C5a-induced chemotaxis preincubated for 30 mins followed... | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C5a anaphylatoxin chemotactic receptor 1 (Homo sapiens (Human)) | BDBM50462093 (CHEMBL4245250) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Antagonist activity at C5aR1 in human blood granulocytes assessed as inhibition of recombinant human C5a-induced CD11b expression on granulocytes pre... | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C5a anaphylatoxin chemotactic receptor 1 (Homo sapiens (Human)) | BDBM50462093 (CHEMBL4245250) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Antagonist activity at C5aR1 in human neutrophils assessed as inhibition of recombinant human C5a-induced chemotaxis preincubated for 30 mins followe... | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C5a anaphylatoxin chemotactic receptor 1 (Homo sapiens (Human)) | BDBM50462093 (CHEMBL4245250) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Antagonist activity at C5aR1 in human U937 cells assessed as inhibition of recombinant human C5a-induced cell degranulation preincubated for 30 mins ... | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C5a anaphylatoxin chemotactic receptor 1 (Homo sapiens (Human)) | BDBM50462093 (CHEMBL4245250) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Antagonist activity at recombinant human C5aR1 expressed in Sf9 insect cell membranes co-expressing G-alphai2,G-beta1 and G-gamma2 assessed as inhibi... | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C5a anaphylatoxin chemotactic receptor 1 (Homo sapiens (Human)) | BDBM50462093 (CHEMBL4245250) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Antagonist activity at C5aR1 in human U937 cells assessed as inhibition of recombinant human C5a-induced intracellular calcium mobilization preincuba... | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1s subcomponent (Homo sapiens (Human)) | BDBM50201566 (CHEMBL2220483) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human C1s esterolytic activity using benzyloxycarbonyl-Lys-thiobenzyl as substrate | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C5a anaphylatoxin chemotactic receptor 1 (Homo sapiens (Human)) | BDBM50462086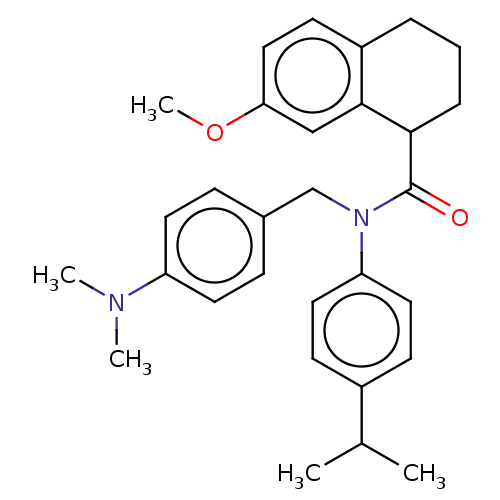 (CHEMBL1628668) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Antagonist activity at C5aR1 in human neutrophils assessed as inhibition of 0.1 nM recombinant human C5a-induced intracellular calcium release | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C5a anaphylatoxin chemotactic receptor 1 (Homo sapiens (Human)) | BDBM50462086 (CHEMBL1628668) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Antagonist activity at C5aR1 in HMDM assessed as inhibition of 1 nM recombinant human C5a-induced intracellular calcium release preincubated for 1 hr... | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM354268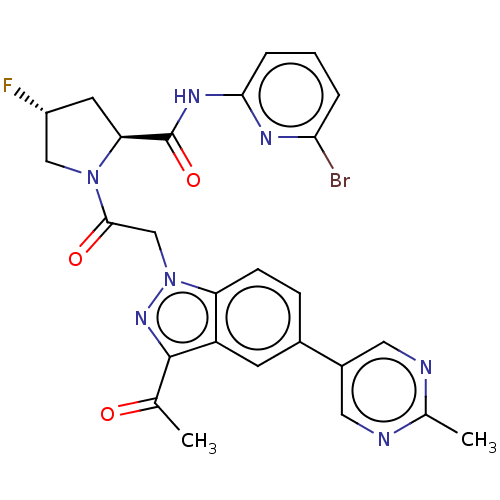 ((2S,4R)-1-(2-(3-acetyl- 5-(2-methylpyrimidin- 5-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of factor D in human serum assessed as decrease in alternative pathway of complement-mediated hemolysis of PNH erythrocytes | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM50462108 (CHEMBL4246585) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human factor D expressed in Escherichia coli using CVF-FB as substrate assessed as decrease in formation of factor B cleavage product B... | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM354268 ((2S,4R)-1-(2-(3-acetyl- 5-(2-methylpyrimidin- 5-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of factor D in human serum assessed as decrease in C3 deposition on PNH erythrocytes | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM354268 ((2S,4R)-1-(2-(3-acetyl- 5-(2-methylpyrimidin- 5-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human factor D using C3b as substrate assessed as decrease in formation of factor B cleavage product Ba | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C5a anaphylatoxin chemotactic receptor 1 (Homo sapiens (Human)) | BDBM50111445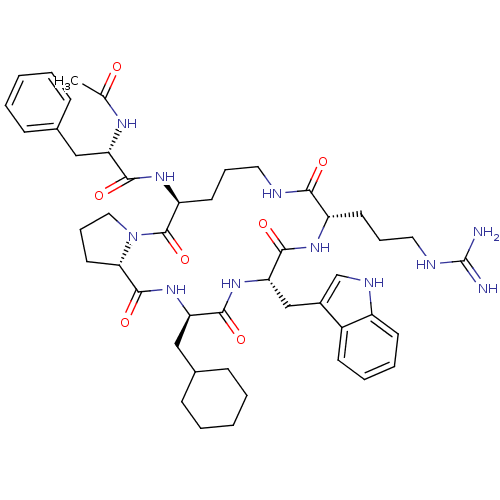 ((S)-N-((3R,6S,9S,15S,20aS)-6-((1H-indol-3-yl)methy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Antagonist activity at C5aR1 in HMDM | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM50462107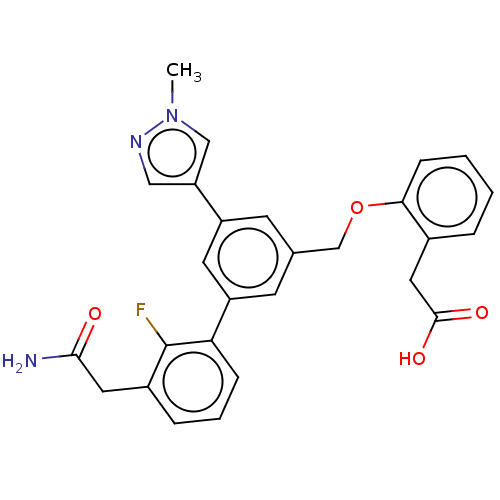 (CHEMBL4249566) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Displacement of Cy5 from recombinant human biotin labeled factor D expressed in Escherichia coli after 2 hrs by TR-FRET assay | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor B (Homo sapiens (Human)) | BDBM50462112 (CHEMBL4247942) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Displacement of Cy5 from recombinant human biotin-labeled factor B expressed in drosophila cells after 2 hrs by TR-FRET assay | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C3a anaphylatoxin chemotactic receptor (Homo sapiens (Human)) | BDBM50322650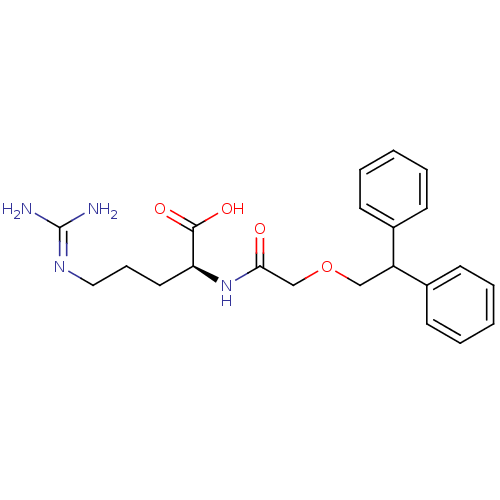 ((S)-2-(2-(2,2-diphenylethoxy)acetamido)-5-guanidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Antagonist activity at human C3aR expressed in RBL-2H3 cells assessed as inhibition of C3a-induced intracellular calcium mobilization | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1s subcomponent (Homo sapiens (Human)) | BDBM50063698 (4-Guanidino-benzoic acid 6-carbamimidoyl-naphthale...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human serum C1s using AGLME as substrate after 30 mins | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM171268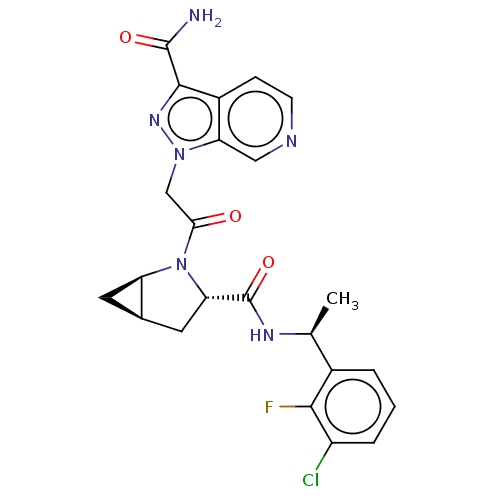 (US9085555, 698) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human factor D using CVF-FB as substrate assessed as decrease in formation of factor B cleavage product Ba by ELISA | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C5a anaphylatoxin chemotactic receptor 1 (Homo sapiens (Human)) | BDBM50111445 ((S)-N-((3R,6S,9S,15S,20aS)-6-((1H-indol-3-yl)methy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Antagonist activity at C5aR1 in human PMN | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor B (Homo sapiens (Human)) | BDBM251882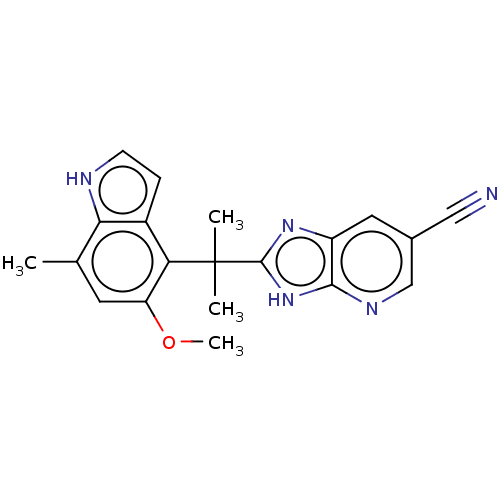 (US9475806, 12-D) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Displacement of Cy5 from recombinant human biotin-labeled factor B expressed in drosophila cells after 2 hrs by TR-FRET assay | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannan-binding lectin serine protease 1 (Homo sapiens) | BDBM50462102 (CHEMBL4240381) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of MASP-1-mediated C3 deposition in human serum after 30 mins by ELISA | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannan-binding lectin serine protease 1 (Homo sapiens) | BDBM50462088 (CHEMBL4250967) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of MASP1 (unknown origin)-induced lectin pathway by ELISA | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1s subcomponent (Homo sapiens (Human)) | BDBM50201566 (CHEMBL2220483) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human serum C1s-mediated lysis of rabbit Ab-sensitized sheep erythrocytes after 60 mins | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C3a anaphylatoxin chemotactic receptor (Homo sapiens (Human)) | BDBM50462095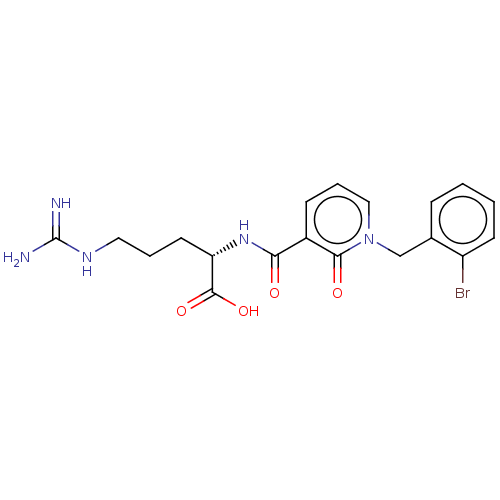 (CHEMBL4239466) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Displacement of 125I-C3a from recombinant human C3aR expressed in HEK293 cell membranes after 3 hrs by scintillation proximity assay | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C3 (Homo sapiens (Human)) | BDBM50462096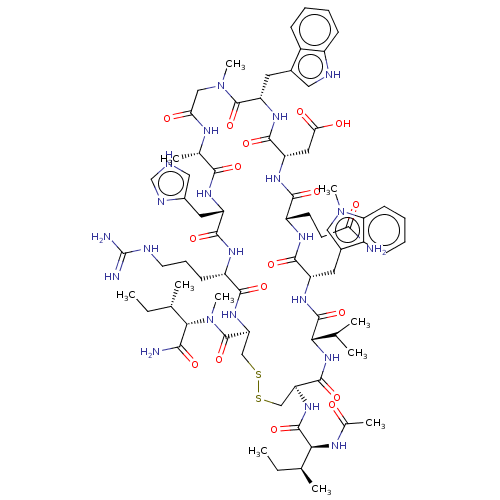 (CHEMBL4247287) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of C3 in human serum by ELISA | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannan-binding lectin serine protease 2 (Homo sapiens) | BDBM50462098 (CHEMBL4242955) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of MASP2 (unknown origin)-induced lectin pathway by ELISA | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM171268 (US9085555, 698) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of factor D in human serum assessed as decrease in lysis of human erythrocytes after 1 hr | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM171268 (US9085555, 698) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of factor D in human serum assessed as decrease in C3 deposition on human erythrocytes after 1 hr | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM50201566 (CHEMBL2220483) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human factor D esterolytic activity using benzyloxycarbonyl-Lys-thiobenzyl as substrate | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Rattus norvegicus) | BDBM50462091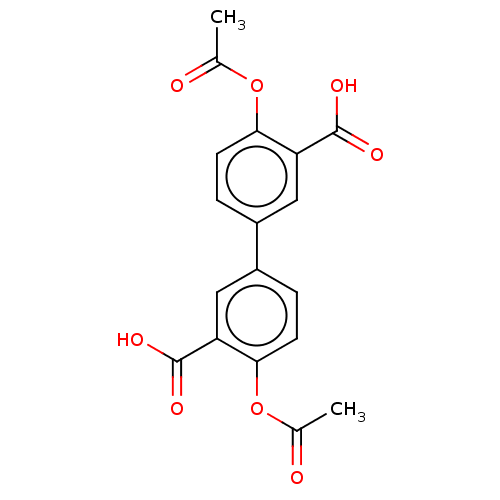 (CHEMBL4241950) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of factor D in zymogen-activated rat serum assessed as decrease in human erythrocyte lysis | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM50462091 (CHEMBL4241950) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of factor D in zymogen-activated human serum assessed as decrease in human erythrocyte lysis | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM50462089 (CHEMBL4243237) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of factor D in zymogen-activated human serum assessed as decrease in human erythrocyte lysis | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Rattus norvegicus) | BDBM50462089 (CHEMBL4243237) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of factor D in zymogen-activated rat serum assessed as decrease in human erythrocyte lysis | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-M/beta-2 (Homo sapiens (Human)) | BDBM50462087 (CHEMBL4243248) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of C3bi-AP binding to CR3 derived from human PMN | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM171268 (US9085555, 698) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 144 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of factor D in human plasma assessed as decrease in alternative pathway-mediated MAC formation preincubated for 30 mins measured after 30 ... | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C3a anaphylatoxin chemotactic receptor (Homo sapiens (Human)) | BDBM50322650 ((S)-2-(2-(2,2-diphenylethoxy)acetamido)-5-guanidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Displacement of 125I-C3a from human C3aR expressed in RBL-2H3 cells | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannan-binding lectin serine protease 1 (Homo sapiens) | BDBM50462099 (CHEMBL4246684) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of MASP-1-mediated C3 deposition in human serum after 30 mins by ELISA | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannan-binding lectin serine protease 2 (Homo sapiens) | BDBM50462102 (CHEMBL4240381) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of MASP-2-mediated C4 deposition in human serum after 1 hr by ELISA | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor B (Homo sapiens (Human)) | BDBM50462109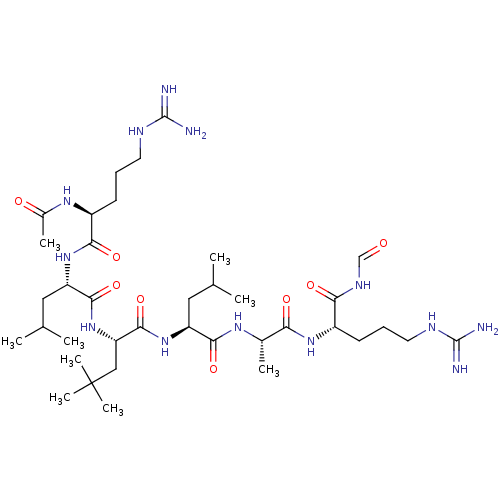 (CHEMBL4244153) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human factor B-induced cleavage of C3 after 3 hrs by coomassie brilliant blue staining-based gel electrophoresis | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C3a anaphylatoxin chemotactic receptor (Homo sapiens (Human)) | BDBM50462104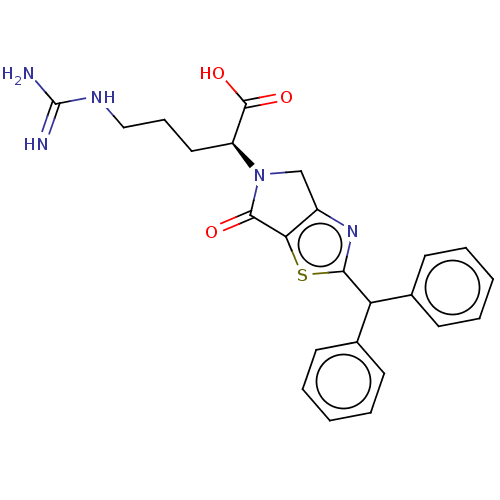 (CHEMBL4248716) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Antagonist activity at C3aR in HMDM assessed as inhibition of agonist-induced Ca2+ release | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-M/beta-2 (Homo sapiens (Human)) | BDBM75309 ((2Z)-3-ethyl-2-[(E)-3-(3-ethyl-1,3-benzothiazol-3-...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of C3bi-AP binding to CR3 derived from human PMN | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C2 (Homo sapiens) | BDBM50462097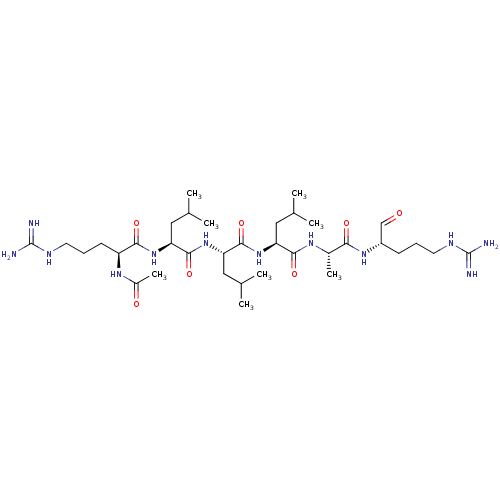 (CHEMBL4247125) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Competitive/reversible inhibition of human C2 using Ac-SHLGLAR-pNA as substrate | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement component C9 (Homo sapiens) | BDBM60996 (5-[(3-carboxy-4-hydroxy-phenyl)-(3-carboxy-4-keto-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of C9 binding to C5b678 in zymogen activated human serum assessed as suppression of human erythrocyte lysis after 1 hr by ELISA | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C2 (Homo sapiens) | BDBM50462097 (CHEMBL4247125) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human C2-mediated C3 processing after 24 hrs by SDS-PAGE | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement component C9 (Homo sapiens) | BDBM50462103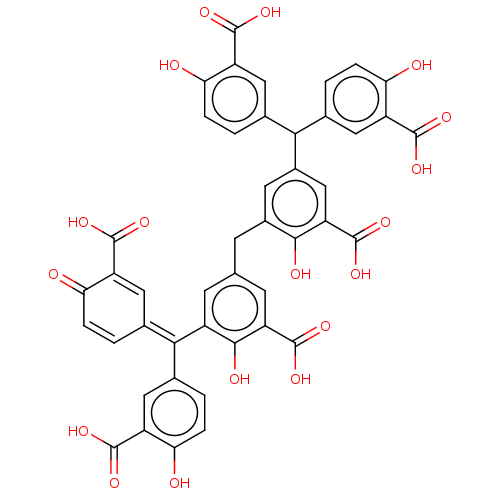 (CHEMBL4238111) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of C9 binding to C5b678 in zymogen activated human serum assessed as suppression of human erythrocyte lysis after 1 hr by ELISA | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement component C9 (Homo sapiens) | BDBM50462092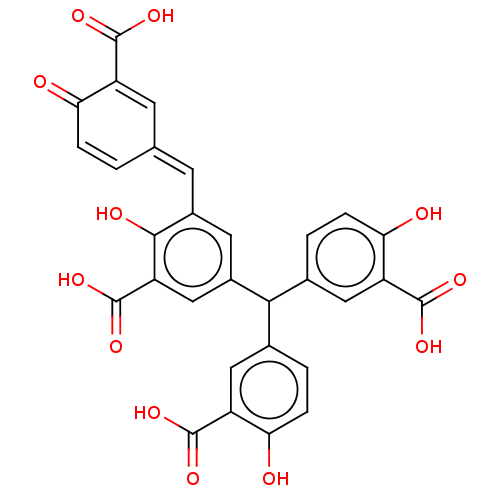 (CHEMBL4242254) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of C9 binding to C5b678 in zymogen activated human serum assessed as suppression of human erythrocyte lysis after 1 hr by ELISA | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50462100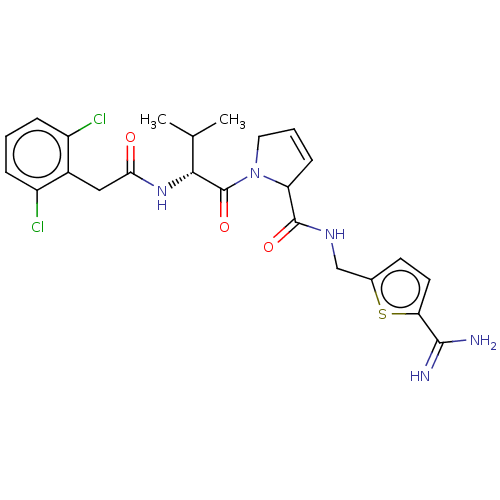 (CHEMBL4247936) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human plasma C1r using Cbz-Gly-Arg-S-Bzl as substrate preincubated for 10 mins followed by substrate addition and measured after 30 min... | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50063698 (4-Guanidino-benzoic acid 6-carbamimidoyl-naphthale...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human serum C1r using AAME as substrate after 30 mins | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1s subcomponent (Homo sapiens (Human)) | BDBM50462100 (CHEMBL4247936) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human plasma C1s using Cbz-Gly-Arg-S-Bzl as substrate preincubated for 10 mins followed by substrate addition and measured after 30 min... | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-M/beta-2 (Homo sapiens (Human)) | BDBM75309 ((2Z)-3-ethyl-2-[(E)-3-(3-ethyl-1,3-benzothiazol-3-...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Antagonist activity at CR3 in carboxyfluorescein diacetate succiminidyl ester-labeled human PMN assessed as inhibition of TNF/PMA-stimulated adhesion... | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-M/beta-2 (Homo sapiens (Human)) | BDBM50462087 (CHEMBL4243248) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Antagonist activity at CR3 in carboxyfluorescein diacetate succiminidyl ester-labeled human PMN assessed as inhibition of TNF/PMA-stimulated adhesion... | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannan-binding lectin serine protease 2 (Homo sapiens) | BDBM50462099 (CHEMBL4246684) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of MASP-2-mediated C4 deposition in human serum after 1 hr by ELISA | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C2 (Homo sapiens) | BDBM50462097 (CHEMBL4247125) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of C2-mediated MAC formation in human serum by ELISA | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C3 (Homo sapiens (Human)) | BDBM50241352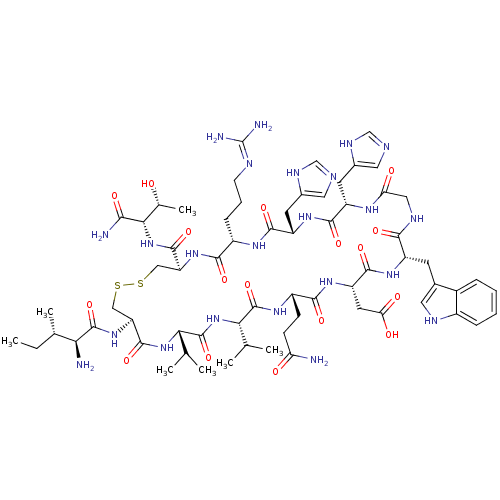 (2-[(4R,7S,10S,13S,19S,22S,25S,28S,31S,34R)-34-[(2S...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of C3 (unknown origin)-binding to convertase of alternative pathway by hemolytic assay | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor B (Homo sapiens (Human)) | BDBM50242426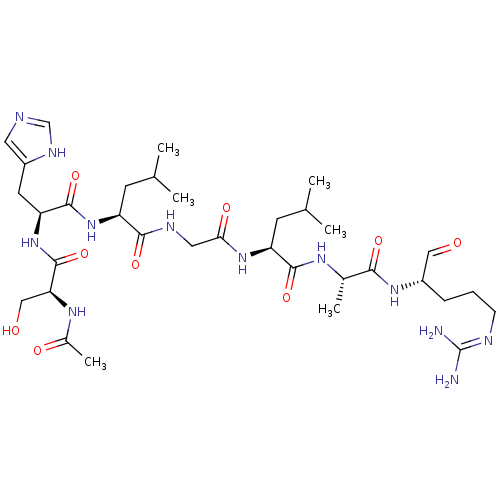 (Ac-SHLGLAR-H | CHEMBL504763) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human factor B-induced cleavage of C3 after 3 hrs by coomassie brilliant blue staining-based gel electrophoresis | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C2 (Homo sapiens) | BDBM50462097 (CHEMBL4247125) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human serum C2-mediated lysis of antibody-sensitized sheep red blood cells after 30 mins by UV-Vis spectrophotometric method | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C3 (Homo sapiens (Human)) | BDBM50241352 (2-[(4R,7S,10S,13S,19S,22S,25S,28S,31S,34R)-34-[(2S...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of C3 (unknown origin)-binding to convertase of classical pathway by hemolytic assay | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM354268 ((2S,4R)-1-(2-(3-acetyl- 5-(2-methylpyrimidin- 5-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Binding affinity to human factor D by SPR analysis | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C3 (Homo sapiens (Human)) | BDBM50462101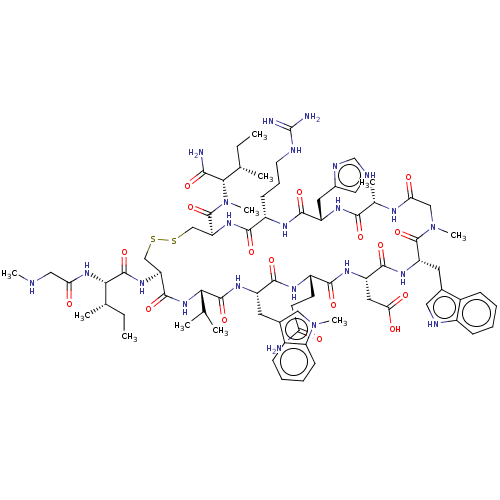 (CHEMBL4241221) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Binding affinity to C3b derived from human plasma by SPR analysis | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C3a anaphylatoxin chemotactic receptor (Homo sapiens (Human)) | BDBM50462106 (CHEMBL3736108) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Agonist activity at C3aR in HMDM assessed as induction of Ca2+ release measured for 5 mins by Fluo-3 AM dye-based fluorescence assay | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C3 (Homo sapiens (Human)) | BDBM50462096 (CHEMBL4247287) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Binding affinity to C3 derived from human plasma by ITC analysis | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C3a anaphylatoxin chemotactic receptor (Homo sapiens (Human)) | BDBM50462105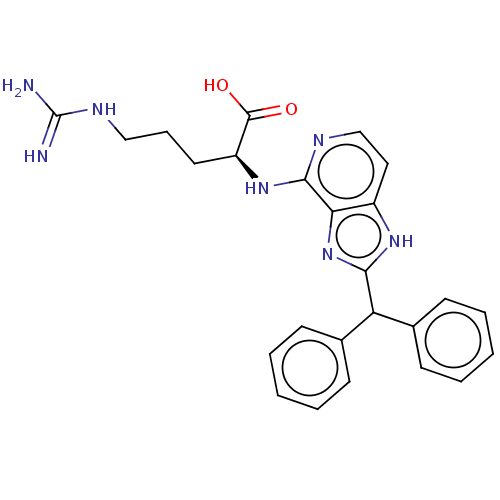 (CHEMBL4239035) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Agonist activity at C3aR in HMDM assessed as induction of Ca2+ release | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||