

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(2) dopamine receptor (BOVINE) | BDBM50020682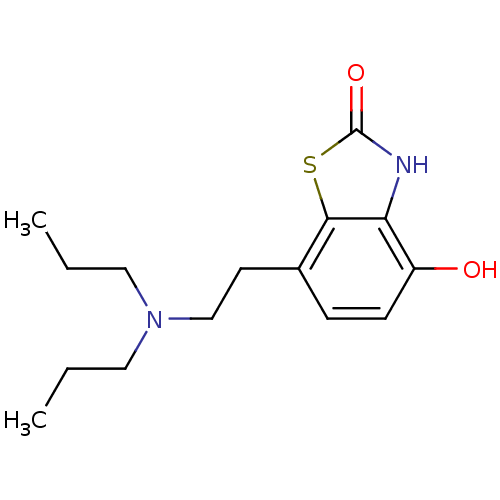 (7-(2-Dipropylamino-ethyl)-4-hydroxy-3H-benzothiazo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-spiroperidol from homogenized bovine pituitary Dopamine receptor D2 | J Med Chem 30: 1166-76 (1987) BindingDB Entry DOI: 10.7270/Q2028QJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50019393 (4-(2-Dipropylamino-ethyl)-7-hydroxy-1,3-dihydro-in...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-spiroperidol from homogenized bovine pituitary Dopamine receptor D2 | J Med Chem 30: 1166-76 (1987) BindingDB Entry DOI: 10.7270/Q2028QJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50020677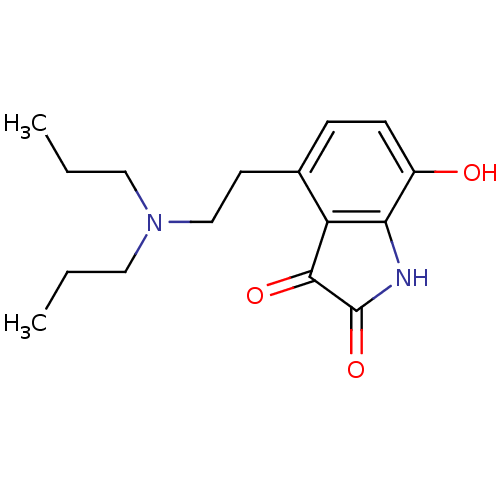 (4-(2-Dipropylamino-ethyl)-7-hydroxy-1H-indole-2,3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-spiroperidol from homogenized bovine pituitary Dopamine receptor D2 | J Med Chem 30: 1166-76 (1987) BindingDB Entry DOI: 10.7270/Q2028QJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50020684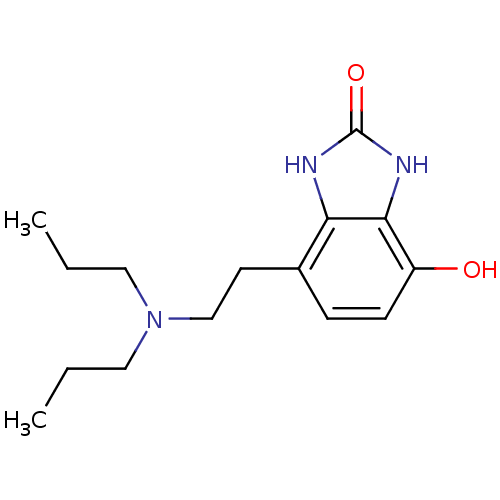 (4-(2-Dipropylamino-ethyl)-7-hydroxy-1,3-dihydro-be...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-spiroperidol from homogenized bovine pituitary Dopamine receptor D2 | J Med Chem 30: 1166-76 (1987) BindingDB Entry DOI: 10.7270/Q2028QJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50020678 (7-(2-Dipropylamino-ethyl)-3H-benzothiazol-2-one | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-spiroperidol from homogenized bovine pituitary Dopamine receptor D2 | J Med Chem 30: 1166-76 (1987) BindingDB Entry DOI: 10.7270/Q2028QJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50020685 (7-(2-Dipropylamino-ethyl)-4-hydroxy-3H-benzooxazol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-spiroperidol from homogenized bovine pituitary Dopamine receptor D2 | J Med Chem 30: 1166-76 (1987) BindingDB Entry DOI: 10.7270/Q2028QJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM60917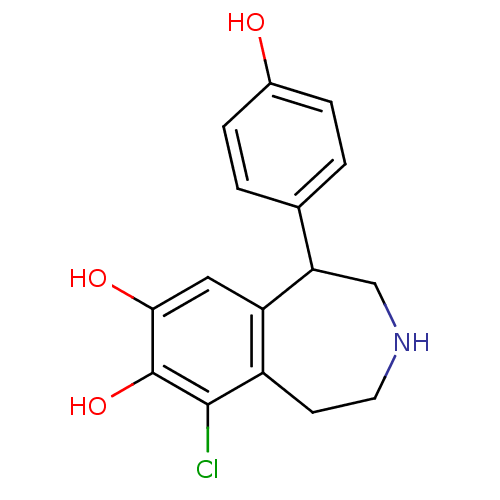 (9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-spiroperidol from homogenized bovine pituitary Dopamine receptor D2 | J Med Chem 30: 1166-76 (1987) BindingDB Entry DOI: 10.7270/Q2028QJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50020679 (7-(2-Amino-ethyl)-4-hydroxy-3H-benzothiazol-2-one ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-spiroperidol from homogenized bovine pituitary Dopamine receptor D2 | J Med Chem 30: 1166-76 (1987) BindingDB Entry DOI: 10.7270/Q2028QJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM55121 (3-HYDROXYTYRAMINE HYDROCHLORIDE | 4-(2-aminoethyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-spiroperidol from homogenized bovine pituitary Dopamine receptor D2 | J Med Chem 30: 1166-76 (1987) BindingDB Entry DOI: 10.7270/Q2028QJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50020686 (4-(2-Amino-ethyl)-7-hydroxy-1,3-dihydro-indol-2-on...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-spiroperidol from homogenized bovine pituitary Dopamine receptor D2 | J Med Chem 30: 1166-76 (1987) BindingDB Entry DOI: 10.7270/Q2028QJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50020680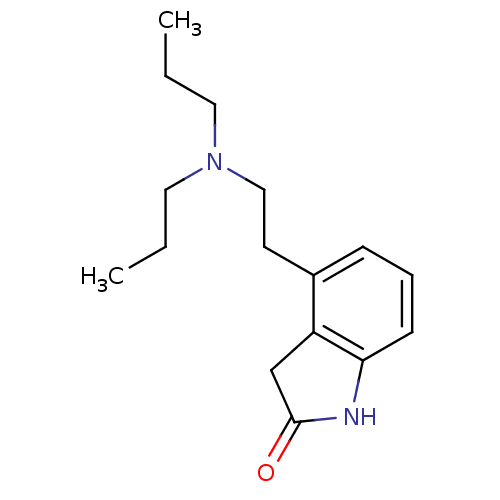 (4-[2-(dipropylamino)ethyl]-1,3-dihydro-2H-indol-2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-spiroperidol from homogenized bovine pituitary Dopamine receptor D2 | J Med Chem 30: 1166-76 (1987) BindingDB Entry DOI: 10.7270/Q2028QJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50020683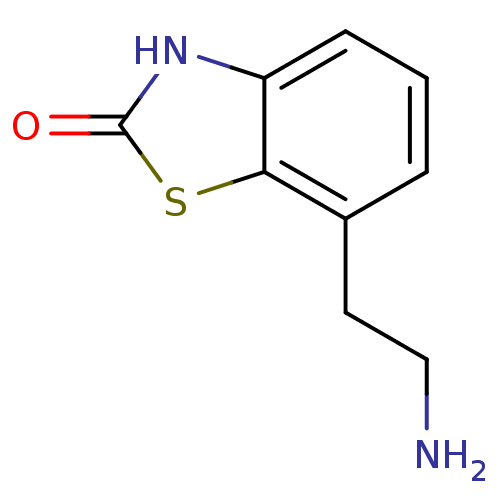 (7-(2-Amino-ethyl)-3H-benzothiazol-2-one | CHEMBL44...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-spiroperidol from homogenized bovine pituitary Dopamine receptor D2 | J Med Chem 30: 1166-76 (1987) BindingDB Entry DOI: 10.7270/Q2028QJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50019393 (4-(2-Dipropylamino-ethyl)-7-hydroxy-1,3-dihydro-in...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Dopamine D1 agonist efficacy was measured with stimulation of dopamine-sensitive rat adenylate cyclase in caudate membranes | J Med Chem 30: 1166-76 (1987) BindingDB Entry DOI: 10.7270/Q2028QJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50020679 (7-(2-Amino-ethyl)-4-hydroxy-3H-benzothiazol-2-one ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 295 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Dopamine receptor D1 agonist efficacy was measured with stimulation of dopamine-sensitive rat adenylate cyclase in caudate membranes. Partial agonist... | J Med Chem 30: 1166-76 (1987) BindingDB Entry DOI: 10.7270/Q2028QJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50020686 (4-(2-Amino-ethyl)-7-hydroxy-1,3-dihydro-indol-2-on...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 4.65E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Dopamine receptor D1 agonist efficacy was measured with stimulation of dopamine-sensitive rat adenylate cyclase in caudate membranes. Partial agonist... | J Med Chem 30: 1166-76 (1987) BindingDB Entry DOI: 10.7270/Q2028QJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50019396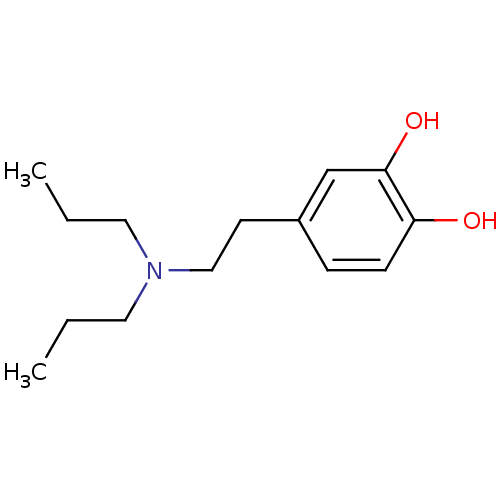 (4-(2-Dipropylamino-ethyl)-benzene-1,2-diol | 4-(2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Dopamine receptor D1 agonist efficacy was measured with stimulation of dopamine-sensitive rat adenylate cyclase in caudate membranes | J Med Chem 30: 1166-76 (1987) BindingDB Entry DOI: 10.7270/Q2028QJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM60917 (9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | n/a | n/a | 57 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Dopamine receptor D1 agonist efficacy was measured with stimulation of dopamine-sensitive rat adenylate cyclase in caudate membranes | J Med Chem 30: 1166-76 (1987) BindingDB Entry DOI: 10.7270/Q2028QJ6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM55121 (3-HYDROXYTYRAMINE HYDROCHLORIDE | 4-(2-aminoethyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Dopamine receptor D1 agonist efficacy was measured with stimulation of dopamine-sensitive rat adenylate cyclase in caudate membranes | J Med Chem 30: 1166-76 (1987) BindingDB Entry DOI: 10.7270/Q2028QJ6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||