 Found 32 hits of Enzyme Inhibition Constant Data
Found 32 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C
(Homo sapiens (Human)) | BDBM50540037
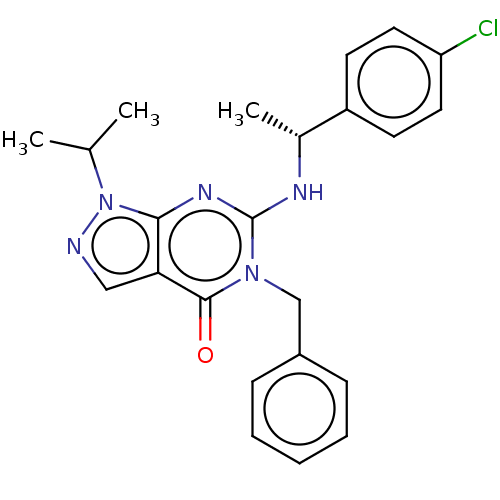
(CHEMBL4639341)Show SMILES CC(C)n1ncc2c1nc(N[C@H](C)c1ccc(Cl)cc1)n(Cc1ccccc1)c2=O |r| Show InChI InChI=1S/C23H24ClN5O/c1-15(2)29-21-20(13-25-29)22(30)28(14-17-7-5-4-6-8-17)23(27-21)26-16(3)18-9-11-19(24)12-10-18/h4-13,15-16H,14H2,1-3H3,(H,26,27)/t16-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE1C2 (147 to 531 residues) (unknown origin) using [3H]-cGMP substrate incubated for 15 mins by liquid scintillation counting method |
J Med Chem 63: 7867-7879 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00711
BindingDB Entry DOI: 10.7270/Q20V8HBZ |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C
(Homo sapiens (Human)) | BDBM50540040

(CHEMBL4640793)Show SMILES C[C@@H](Nc1nc2n(ncc2c(=O)n1Cc1ccccc1)C(C)(C)C)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C24H26ClN5O/c1-16(18-10-12-19(25)13-11-18)27-23-28-21-20(14-26-30(21)24(2,3)4)22(31)29(23)15-17-8-6-5-7-9-17/h5-14,16H,15H2,1-4H3,(H,27,28)/t16-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE1C2 (147 to 531 residues) (unknown origin) using [3H]-cGMP substrate incubated for 15 mins by liquid scintillation counting method |
J Med Chem 63: 7867-7879 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00711
BindingDB Entry DOI: 10.7270/Q20V8HBZ |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C
(Homo sapiens (Human)) | BDBM50540042
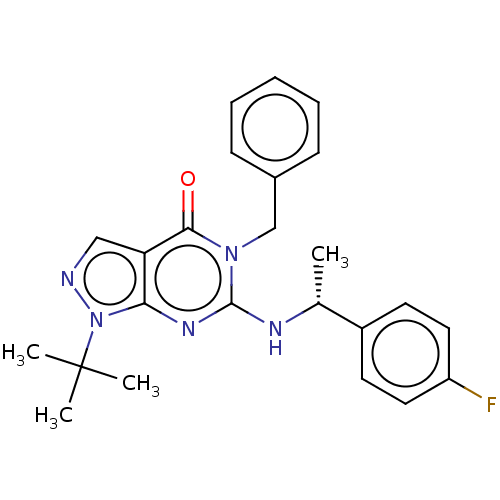
(CHEMBL4647278)Show SMILES C[C@@H](Nc1nc2n(ncc2c(=O)n1Cc1ccccc1)C(C)(C)C)c1ccc(F)cc1 |r| Show InChI InChI=1S/C24H26FN5O/c1-16(18-10-12-19(25)13-11-18)27-23-28-21-20(14-26-30(21)24(2,3)4)22(31)29(23)15-17-8-6-5-7-9-17/h5-14,16H,15H2,1-4H3,(H,27,28)/t16-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE1C2 (147 to 531 residues) (unknown origin) using [3H]-cGMP substrate incubated for 15 mins by liquid scintillation counting method |
J Med Chem 63: 7867-7879 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00711
BindingDB Entry DOI: 10.7270/Q20V8HBZ |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C
(Homo sapiens (Human)) | BDBM50540044
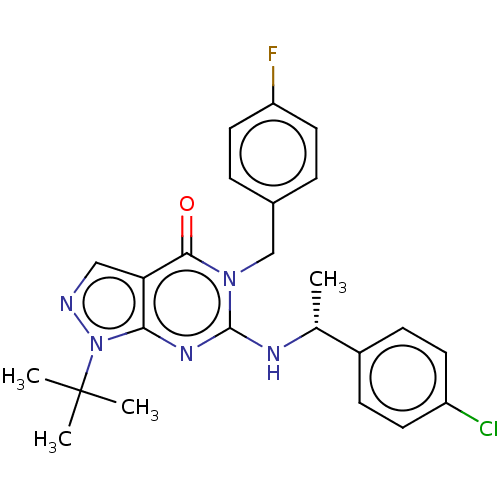
(CHEMBL4644729)Show SMILES C[C@@H](Nc1nc2n(ncc2c(=O)n1Cc1ccc(F)cc1)C(C)(C)C)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C24H25ClFN5O/c1-15(17-7-9-18(25)10-8-17)28-23-29-21-20(13-27-31(21)24(2,3)4)22(32)30(23)14-16-5-11-19(26)12-6-16/h5-13,15H,14H2,1-4H3,(H,28,29)/t15-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE1C2 (147 to 531 residues) (unknown origin) using [3H]-cGMP substrate incubated for 15 mins by liquid scintillation counting method |
J Med Chem 63: 7867-7879 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00711
BindingDB Entry DOI: 10.7270/Q20V8HBZ |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1B
(Homo sapiens (Human)) | BDBM50540044

(CHEMBL4644729)Show SMILES C[C@@H](Nc1nc2n(ncc2c(=O)n1Cc1ccc(F)cc1)C(C)(C)C)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C24H25ClFN5O/c1-15(17-7-9-18(25)10-8-17)28-23-29-21-20(13-27-31(21)24(2,3)4)22(32)30(23)14-16-5-11-19(26)12-6-16/h5-13,15H,14H2,1-4H3,(H,28,29)/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE1B (146 to 506 residues) (unknown origin) using [3H]-cGMP substrate incubated for 15 mins by liquid scintillation counting method |
J Med Chem 63: 7867-7879 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00711
BindingDB Entry DOI: 10.7270/Q20V8HBZ |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C
(Homo sapiens (Human)) | BDBM50540035
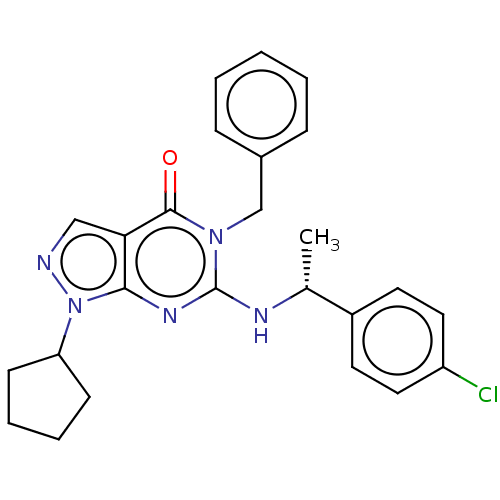
(CHEMBL4649493)Show SMILES C[C@@H](Nc1nc2n(ncc2c(=O)n1Cc1ccccc1)C1CCCC1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C25H26ClN5O/c1-17(19-11-13-20(26)14-12-19)28-25-29-23-22(15-27-31(23)21-9-5-6-10-21)24(32)30(25)16-18-7-3-2-4-8-18/h2-4,7-8,11-15,17,21H,5-6,9-10,16H2,1H3,(H,28,29)/t17-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE1C2 (147 to 531 residues) (unknown origin) using [3H]-cGMP substrate incubated for 15 mins by liquid scintillation counting method |
J Med Chem 63: 7867-7879 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00711
BindingDB Entry DOI: 10.7270/Q20V8HBZ |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C
(Homo sapiens (Human)) | BDBM50540039
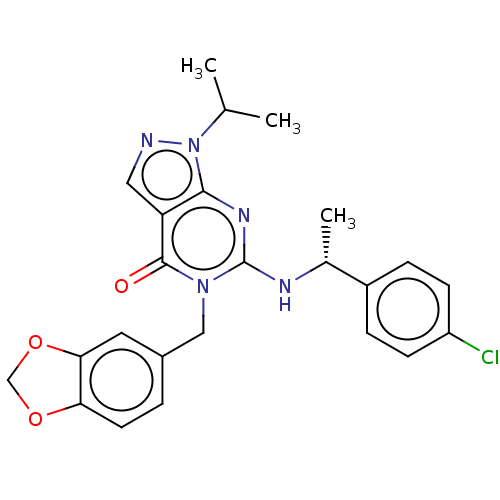
(CHEMBL4639286)Show SMILES CC(C)n1ncc2c1nc(N[C@H](C)c1ccc(Cl)cc1)n(Cc1ccc3OCOc3c1)c2=O |r| Show InChI InChI=1S/C24H24ClN5O3/c1-14(2)30-22-19(11-26-30)23(31)29(12-16-4-9-20-21(10-16)33-13-32-20)24(28-22)27-15(3)17-5-7-18(25)8-6-17/h4-11,14-15H,12-13H2,1-3H3,(H,27,28)/t15-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE1C2 (147 to 531 residues) (unknown origin) using [3H]-cGMP substrate incubated for 15 mins by liquid scintillation counting method |
J Med Chem 63: 7867-7879 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00711
BindingDB Entry DOI: 10.7270/Q20V8HBZ |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1B
(Homo sapiens (Human)) | BDBM50540035

(CHEMBL4649493)Show SMILES C[C@@H](Nc1nc2n(ncc2c(=O)n1Cc1ccccc1)C1CCCC1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C25H26ClN5O/c1-17(19-11-13-20(26)14-12-19)28-25-29-23-22(15-27-31(23)21-9-5-6-10-21)24(32)30(25)16-18-7-3-2-4-8-18/h2-4,7-8,11-15,17,21H,5-6,9-10,16H2,1H3,(H,28,29)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE1B (146 to 506 residues) (unknown origin) using [3H]-cGMP substrate incubated for 15 mins by liquid scintillation counting method |
J Med Chem 63: 7867-7879 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00711
BindingDB Entry DOI: 10.7270/Q20V8HBZ |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C
(Homo sapiens (Human)) | BDBM50540046
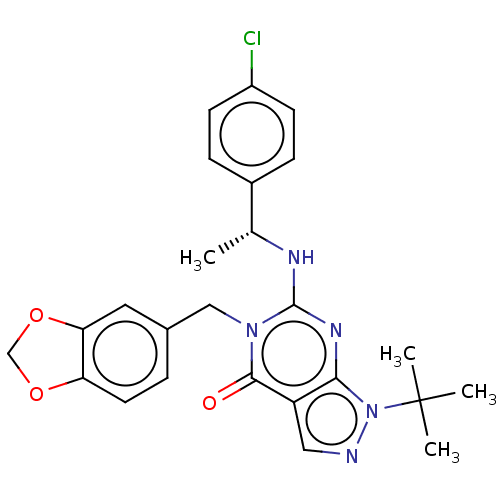
(CHEMBL4638381)Show SMILES C[C@@H](Nc1nc2n(ncc2c(=O)n1Cc1ccc2OCOc2c1)C(C)(C)C)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C25H26ClN5O3/c1-15(17-6-8-18(26)9-7-17)28-24-29-22-19(12-27-31(22)25(2,3)4)23(32)30(24)13-16-5-10-20-21(11-16)34-14-33-20/h5-12,15H,13-14H2,1-4H3,(H,28,29)/t15-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE1C2 (147 to 531 residues) (unknown origin) using [3H]-cGMP substrate incubated for 15 mins by liquid scintillation counting method |
J Med Chem 63: 7867-7879 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00711
BindingDB Entry DOI: 10.7270/Q20V8HBZ |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C
(Homo sapiens (Human)) | BDBM50540038
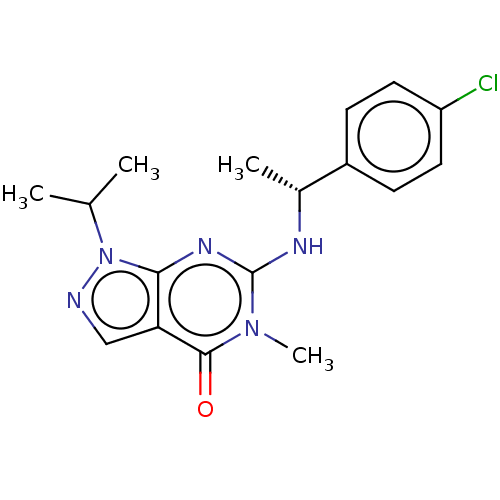
(CHEMBL4642557)Show SMILES CC(C)n1ncc2c1nc(N[C@H](C)c1ccc(Cl)cc1)n(C)c2=O |r| Show InChI InChI=1S/C17H20ClN5O/c1-10(2)23-15-14(9-19-23)16(24)22(4)17(21-15)20-11(3)12-5-7-13(18)8-6-12/h5-11H,1-4H3,(H,20,21)/t11-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE1C2 (147 to 531 residues) (unknown origin) using [3H]-cGMP substrate incubated for 15 mins by liquid scintillation counting method |
J Med Chem 63: 7867-7879 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00711
BindingDB Entry DOI: 10.7270/Q20V8HBZ |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50540047

(CHEMBL4644938)Show SMILES C[C@@H](Nc1nc2n(ncc2c(=O)[nH]1)C1CCCC1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C18H20ClN5O/c1-11(12-6-8-13(19)9-7-12)21-18-22-16-15(17(25)23-18)10-20-24(16)14-4-2-3-5-14/h6-11,14H,2-5H2,1H3,(H2,21,22,23,25)/t11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE9A (181 to 506 residues) (unknown origin) using [3H]-cGMP substrate incubated for 15 mins by liquid scintillation counting method |
J Med Chem 63: 7867-7879 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00711
BindingDB Entry DOI: 10.7270/Q20V8HBZ |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C
(Homo sapiens (Human)) | BDBM50540045
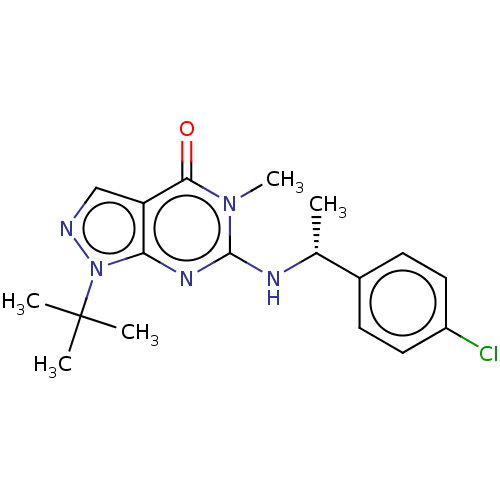
(CHEMBL4634240)Show SMILES C[C@@H](Nc1nc2n(ncc2c(=O)n1C)C(C)(C)C)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C18H22ClN5O/c1-11(12-6-8-13(19)9-7-12)21-17-22-15-14(16(25)23(17)5)10-20-24(15)18(2,3)4/h6-11H,1-5H3,(H,21,22)/t11-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE1C2 (147 to 531 residues) (unknown origin) using [3H]-cGMP substrate incubated for 15 mins by liquid scintillation counting method |
J Med Chem 63: 7867-7879 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00711
BindingDB Entry DOI: 10.7270/Q20V8HBZ |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1B
(Homo sapiens (Human)) | BDBM50540047

(CHEMBL4644938)Show SMILES C[C@@H](Nc1nc2n(ncc2c(=O)[nH]1)C1CCCC1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C18H20ClN5O/c1-11(12-6-8-13(19)9-7-12)21-18-22-16-15(17(25)23-18)10-20-24(16)14-4-2-3-5-14/h6-11,14H,2-5H2,1H3,(H2,21,22,23,25)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE1B (146 to 506 residues) (unknown origin) using [3H]-cGMP substrate incubated for 15 mins by liquid scintillation counting method |
J Med Chem 63: 7867-7879 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00711
BindingDB Entry DOI: 10.7270/Q20V8HBZ |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C
(Homo sapiens (Human)) | BDBM50540041
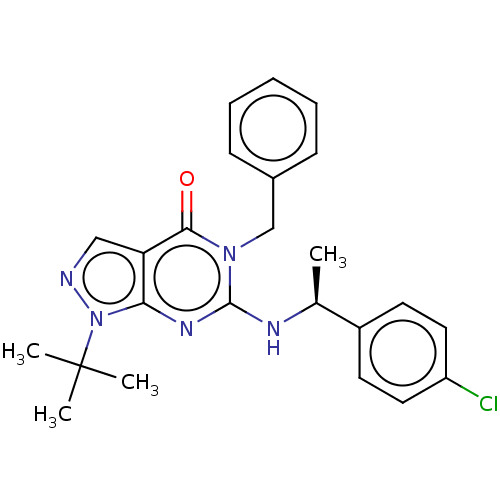
(CHEMBL4632429)Show SMILES C[C@H](Nc1nc2n(ncc2c(=O)n1Cc1ccccc1)C(C)(C)C)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C24H26ClN5O/c1-16(18-10-12-19(25)13-11-18)27-23-28-21-20(14-26-30(21)24(2,3)4)22(31)29(23)15-17-8-6-5-7-9-17/h5-14,16H,15H2,1-4H3,(H,27,28)/t16-/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 157 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE1C2 (147 to 531 residues) (unknown origin) using [3H]-cGMP substrate incubated for 15 mins by liquid scintillation counting method |
J Med Chem 63: 7867-7879 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00711
BindingDB Entry DOI: 10.7270/Q20V8HBZ |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C
(Homo sapiens (Human)) | BDBM50540043
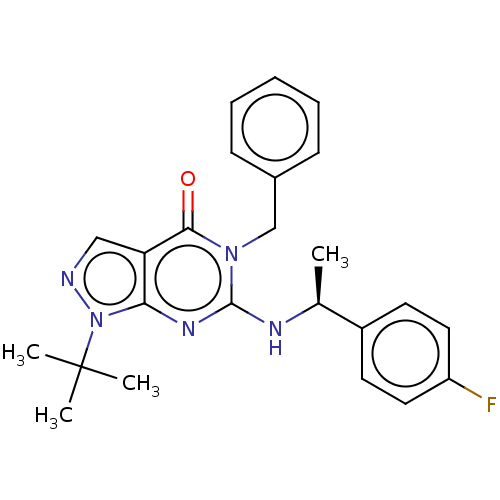
(CHEMBL4636141)Show SMILES C[C@H](Nc1nc2n(ncc2c(=O)n1Cc1ccccc1)C(C)(C)C)c1ccc(F)cc1 |r| Show InChI InChI=1S/C24H26FN5O/c1-16(18-10-12-19(25)13-11-18)27-23-28-21-20(14-26-30(21)24(2,3)4)22(31)29(23)15-17-8-6-5-7-9-17/h5-14,16H,15H2,1-4H3,(H,27,28)/t16-/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 245 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE1C2 (147 to 531 residues) (unknown origin) using [3H]-cGMP substrate incubated for 15 mins by liquid scintillation counting method |
J Med Chem 63: 7867-7879 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00711
BindingDB Entry DOI: 10.7270/Q20V8HBZ |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C
(Homo sapiens (Human)) | BDBM50540036
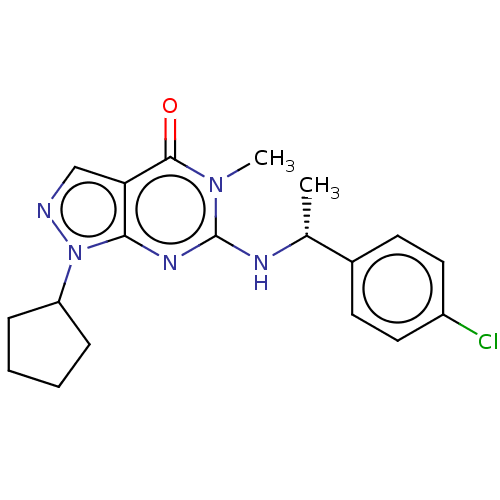
(CHEMBL4632723)Show SMILES C[C@@H](Nc1nc2n(ncc2c(=O)n1C)C1CCCC1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C19H22ClN5O/c1-12(13-7-9-14(20)10-8-13)22-19-23-17-16(18(26)24(19)2)11-21-25(17)15-5-3-4-6-15/h7-12,15H,3-6H2,1-2H3,(H,22,23)/t12-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 258 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE1C2 (147 to 531 residues) (unknown origin) using [3H]-cGMP substrate incubated for 15 mins by liquid scintillation counting method |
J Med Chem 63: 7867-7879 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00711
BindingDB Entry DOI: 10.7270/Q20V8HBZ |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50540044

(CHEMBL4644729)Show SMILES C[C@@H](Nc1nc2n(ncc2c(=O)n1Cc1ccc(F)cc1)C(C)(C)C)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C24H25ClFN5O/c1-15(17-7-9-18(25)10-8-17)28-23-29-21-20(13-27-31(21)24(2,3)4)22(32)30(23)14-16-5-11-19(26)12-6-16/h5-13,15H,14H2,1-4H3,(H,28,29)/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE5A1 (535 to 860 residues) (unknown origin) using [3H]-cGMP substrate incubated for 15 mins by liquid scintillation counting method r... |
J Med Chem 63: 7867-7879 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00711
BindingDB Entry DOI: 10.7270/Q20V8HBZ |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50540044

(CHEMBL4644729)Show SMILES C[C@@H](Nc1nc2n(ncc2c(=O)n1Cc1ccc(F)cc1)C(C)(C)C)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C24H25ClFN5O/c1-15(17-7-9-18(25)10-8-17)28-23-29-21-20(13-27-31(21)24(2,3)4)22(32)30(23)14-16-5-11-19(26)12-6-16/h5-13,15H,14H2,1-4H3,(H,28,29)/t15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 699 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE4D2 (86 to 413 residues) (unknown origin) using [3H]-cAMP substrate incubated for 15 mins by liquid scintillation counting method re... |
J Med Chem 63: 7867-7879 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00711
BindingDB Entry DOI: 10.7270/Q20V8HBZ |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50540044

(CHEMBL4644729)Show SMILES C[C@@H](Nc1nc2n(ncc2c(=O)n1Cc1ccc(F)cc1)C(C)(C)C)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C24H25ClFN5O/c1-15(17-7-9-18(25)10-8-17)28-23-29-21-20(13-27-31(21)24(2,3)4)22(32)30(23)14-16-5-11-19(26)12-6-16/h5-13,15H,14H2,1-4H3,(H,28,29)/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (449 to 770 residues) (unknown origin) using [3H]-cAMP substrate incubated for 15 mins by liquid scintillation counting method r... |
J Med Chem 63: 7867-7879 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00711
BindingDB Entry DOI: 10.7270/Q20V8HBZ |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50540044

(CHEMBL4644729)Show SMILES C[C@@H](Nc1nc2n(ncc2c(=O)n1Cc1ccc(F)cc1)C(C)(C)C)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C24H25ClFN5O/c1-15(17-7-9-18(25)10-8-17)28-23-29-21-20(13-27-31(21)24(2,3)4)22(32)30(23)14-16-5-11-19(26)12-6-16/h5-13,15H,14H2,1-4H3,(H,28,29)/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE8A1 (480 to 820 residues) (unknown origin) using [3H]-cAMP substrate incubated for 15 mins by liquid scintillation counting method r... |
J Med Chem 63: 7867-7879 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00711
BindingDB Entry DOI: 10.7270/Q20V8HBZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50540044

(CHEMBL4644729)Show SMILES C[C@@H](Nc1nc2n(ncc2c(=O)n1Cc1ccc(F)cc1)C(C)(C)C)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C24H25ClFN5O/c1-15(17-7-9-18(25)10-8-17)28-23-29-21-20(13-27-31(21)24(2,3)4)22(32)30(23)14-16-5-11-19(26)12-6-16/h5-13,15H,14H2,1-4H3,(H,28,29)/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 |
J Med Chem 63: 7867-7879 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00711
BindingDB Entry DOI: 10.7270/Q20V8HBZ |
More data for this
Ligand-Target Pair | |
Cone cGMP-specific 3',5'-cyclic phosphodiesterase subunit alpha'
(Homo sapiens (Human)) | BDBM50540044

(CHEMBL4644729)Show SMILES C[C@@H](Nc1nc2n(ncc2c(=O)n1Cc1ccc(F)cc1)C(C)(C)C)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C24H25ClFN5O/c1-15(17-7-9-18(25)10-8-17)28-23-29-21-20(13-27-31(21)24(2,3)4)22(32)30(23)14-16-5-11-19(26)12-6-16/h5-13,15H,14H2,1-4H3,(H,28,29)/t15-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE6C (1 to 858 residues) (unknown origin) using [3H]-cGMP substrate incubated for 15 mins by liquid scintillation counting method rela... |
J Med Chem 63: 7867-7879 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00711
BindingDB Entry DOI: 10.7270/Q20V8HBZ |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50540035

(CHEMBL4649493)Show SMILES C[C@@H](Nc1nc2n(ncc2c(=O)n1Cc1ccccc1)C1CCCC1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C25H26ClN5O/c1-17(19-11-13-20(26)14-12-19)28-25-29-23-22(15-27-31(23)21-9-5-6-10-21)24(32)30(25)16-18-7-3-2-4-8-18/h2-4,7-8,11-15,17,21H,5-6,9-10,16H2,1H3,(H,28,29)/t17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE9A (181 to 506 residues) (unknown origin) using [3H]-cGMP substrate incubated for 15 mins by liquid scintillation counting method |
J Med Chem 63: 7867-7879 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00711
BindingDB Entry DOI: 10.7270/Q20V8HBZ |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50540044

(CHEMBL4644729)Show SMILES C[C@@H](Nc1nc2n(ncc2c(=O)n1Cc1ccc(F)cc1)C(C)(C)C)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C24H25ClFN5O/c1-15(17-7-9-18(25)10-8-17)28-23-29-21-20(13-27-31(21)24(2,3)4)22(32)30(23)14-16-5-11-19(26)12-6-16/h5-13,15H,14H2,1-4H3,(H,28,29)/t15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE2A (580 to 919 residues) (unknown origin) using [3H]-cGMP substrate incubated for 15 mins by liquid scintillation counting method re... |
J Med Chem 63: 7867-7879 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00711
BindingDB Entry DOI: 10.7270/Q20V8HBZ |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A
(Homo sapiens (Human)) | BDBM50540044

(CHEMBL4644729)Show SMILES C[C@@H](Nc1nc2n(ncc2c(=O)n1Cc1ccc(F)cc1)C(C)(C)C)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C24H25ClFN5O/c1-15(17-7-9-18(25)10-8-17)28-23-29-21-20(13-27-31(21)24(2,3)4)22(32)30(23)14-16-5-11-19(26)12-6-16/h5-13,15H,14H2,1-4H3,(H,28,29)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE3A (679 to 1087 residues) (unknown origin) using [3H]-cAMP substrate incubated for 15 mins by liquid scintillation counting method r... |
J Med Chem 63: 7867-7879 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00711
BindingDB Entry DOI: 10.7270/Q20V8HBZ |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50540044

(CHEMBL4644729)Show SMILES C[C@@H](Nc1nc2n(ncc2c(=O)n1Cc1ccc(F)cc1)C(C)(C)C)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C24H25ClFN5O/c1-15(17-7-9-18(25)10-8-17)28-23-29-21-20(13-27-31(21)24(2,3)4)22(32)30(23)14-16-5-11-19(26)12-6-16/h5-13,15H,14H2,1-4H3,(H,28,29)/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE7A1 (130 to 482 residues) (unknown origin) using [3H]-cAMP substrate incubated for 15 mins by liquid scintillation counting method r... |
J Med Chem 63: 7867-7879 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00711
BindingDB Entry DOI: 10.7270/Q20V8HBZ |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50540044

(CHEMBL4644729)Show SMILES C[C@@H](Nc1nc2n(ncc2c(=O)n1Cc1ccc(F)cc1)C(C)(C)C)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C24H25ClFN5O/c1-15(17-7-9-18(25)10-8-17)28-23-29-21-20(13-27-31(21)24(2,3)4)22(32)30(23)14-16-5-11-19(26)12-6-16/h5-13,15H,14H2,1-4H3,(H,28,29)/t15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE9A2 (181 to 506 residues) (unknown origin) using [3H]-cGMP substrate incubated for 15 mins by liquid scintillation counting method r... |
J Med Chem 63: 7867-7879 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00711
BindingDB Entry DOI: 10.7270/Q20V8HBZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50540044

(CHEMBL4644729)Show SMILES C[C@@H](Nc1nc2n(ncc2c(=O)n1Cc1ccc(F)cc1)C(C)(C)C)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C24H25ClFN5O/c1-15(17-7-9-18(25)10-8-17)28-23-29-21-20(13-27-31(21)24(2,3)4)22(32)30(23)14-16-5-11-19(26)12-6-16/h5-13,15H,14H2,1-4H3,(H,28,29)/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 63: 7867-7879 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00711
BindingDB Entry DOI: 10.7270/Q20V8HBZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50540044

(CHEMBL4644729)Show SMILES C[C@@H](Nc1nc2n(ncc2c(=O)n1Cc1ccc(F)cc1)C(C)(C)C)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C24H25ClFN5O/c1-15(17-7-9-18(25)10-8-17)28-23-29-21-20(13-27-31(21)24(2,3)4)22(32)30(23)14-16-5-11-19(26)12-6-16/h5-13,15H,14H2,1-4H3,(H,28,29)/t15-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
J Med Chem 63: 7867-7879 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00711
BindingDB Entry DOI: 10.7270/Q20V8HBZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50540044

(CHEMBL4644729)Show SMILES C[C@@H](Nc1nc2n(ncc2c(=O)n1Cc1ccc(F)cc1)C(C)(C)C)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C24H25ClFN5O/c1-15(17-7-9-18(25)10-8-17)28-23-29-21-20(13-27-31(21)24(2,3)4)22(32)30(23)14-16-5-11-19(26)12-6-16/h5-13,15H,14H2,1-4H3,(H,28,29)/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 using testosterone as substrate |
J Med Chem 63: 7867-7879 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00711
BindingDB Entry DOI: 10.7270/Q20V8HBZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50540044

(CHEMBL4644729)Show SMILES C[C@@H](Nc1nc2n(ncc2c(=O)n1Cc1ccc(F)cc1)C(C)(C)C)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C24H25ClFN5O/c1-15(17-7-9-18(25)10-8-17)28-23-29-21-20(13-27-31(21)24(2,3)4)22(32)30(23)14-16-5-11-19(26)12-6-16/h5-13,15H,14H2,1-4H3,(H,28,29)/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 |
J Med Chem 63: 7867-7879 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00711
BindingDB Entry DOI: 10.7270/Q20V8HBZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50540044

(CHEMBL4644729)Show SMILES C[C@@H](Nc1nc2n(ncc2c(=O)n1Cc1ccc(F)cc1)C(C)(C)C)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C24H25ClFN5O/c1-15(17-7-9-18(25)10-8-17)28-23-29-21-20(13-27-31(21)24(2,3)4)22(32)30(23)14-16-5-11-19(26)12-6-16/h5-13,15H,14H2,1-4H3,(H,28,29)/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 using midazolam as substrate |
J Med Chem 63: 7867-7879 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00711
BindingDB Entry DOI: 10.7270/Q20V8HBZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data