

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50405400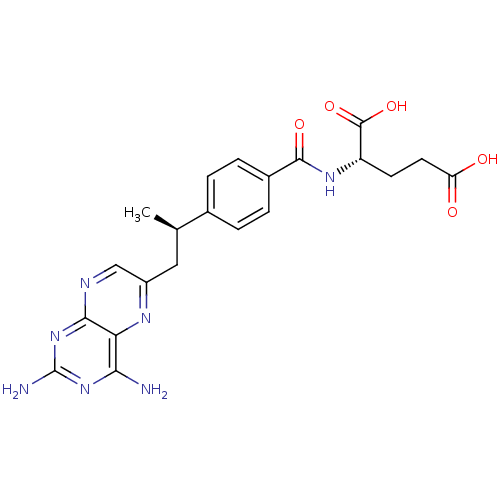 (CHEMBL2051987) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against Dihydrofolate reductase of L1210 cells | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50405400 (CHEMBL2051987) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against Dihydrofolate reductase of L1210 cells | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50226274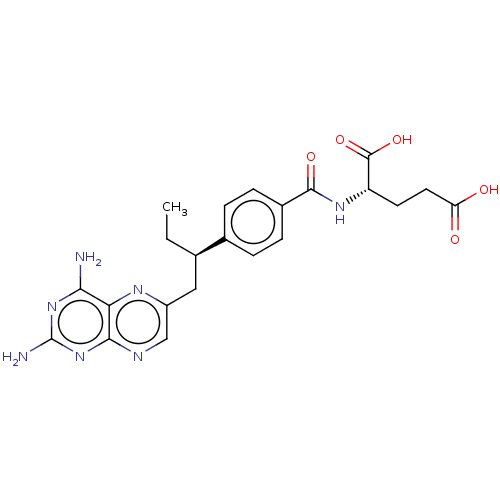 (CHEMBL3349020) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against Dihydrofolate reductase of L1210 cells | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against Dihydrofolate reductase of L1210 cells | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50405401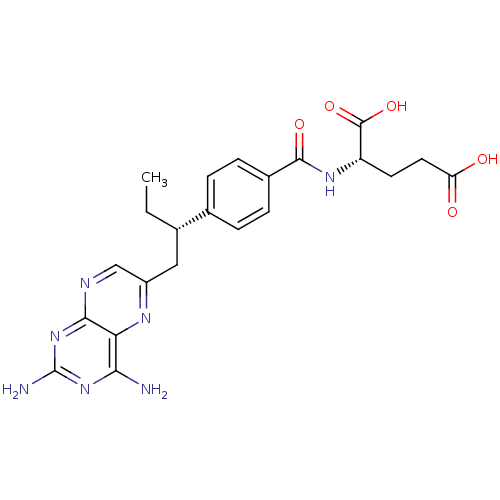 (CHEMBL2051990) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against Dihydrofolate reductase of L1210 cells | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dihydrofolate reductase (DHFR) derived from Lactobacillus casei ATCC 7469 | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50226274 (CHEMBL3349020) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dihydrofolate reductase (DHFR) derived from Lactobacillus casei ATCC 7469 | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Gallus gallus (Chicken)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dihydrofolate reductase (DHFR) derived from Chicken liver | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Gallus gallus (Chicken)) | BDBM50226274 (CHEMBL3349020) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dihydrofolate reductase (DHFR) derived from Chicken liver | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Gallus gallus (Chicken)) | BDBM50405399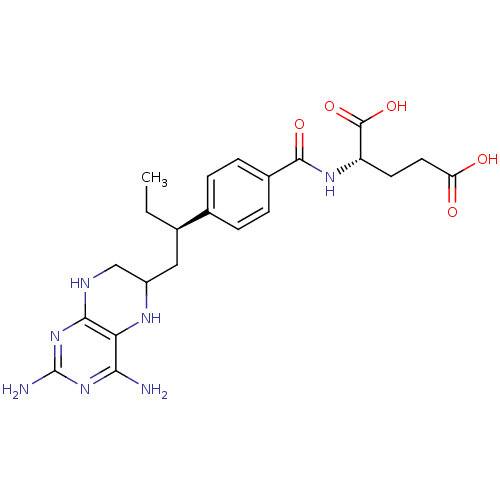 (CHEMBL2051989) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dihydrofolate reductase (DHFR) derived from Chicken liver | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Gallus gallus (Chicken)) | BDBM50004544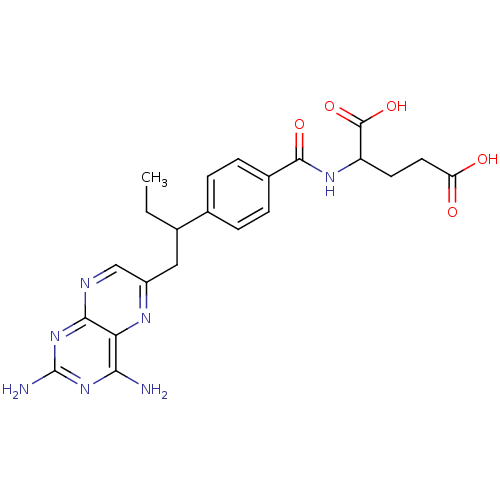 (2-{4-[1-(2,4-Diamino-pteridin-6-ylmethyl)-propyl]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dihydrofolate reductase (DHFR) derived from Chicken liver | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Gallus gallus (Chicken)) | BDBM50405399 (CHEMBL2051989) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dihydrofolate reductase (DHFR) derived from Chicken liver | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50004544 (2-{4-[1-(2,4-Diamino-pteridin-6-ylmethyl)-propyl]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dihydrofolate reductase (DHFR) derived from Lactobacillus casei ATCC 7469 | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Dihydrofolate reductase (Gallus gallus (Chicken)) | BDBM50405401 (CHEMBL2051990) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dihydrofolate reductase (DHFR) derived from Chicken liver | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50025007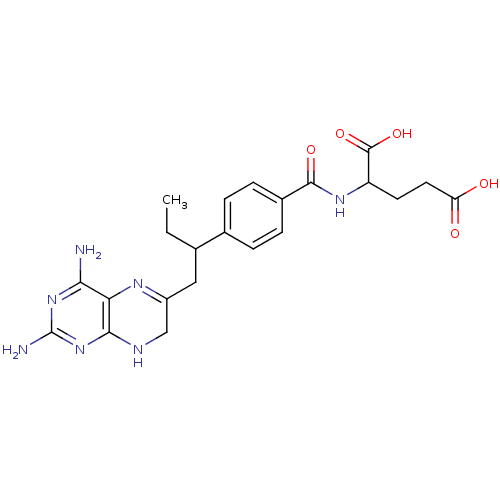 (2-{4-[2-(2,4-Diamino-7,8-dihydro-pteridin-6-yl)-et...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dihydrofolate reductase (DHFR) derived from Lactobacillus casei ATCC 7469 | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Gallus gallus (Chicken)) | BDBM50025008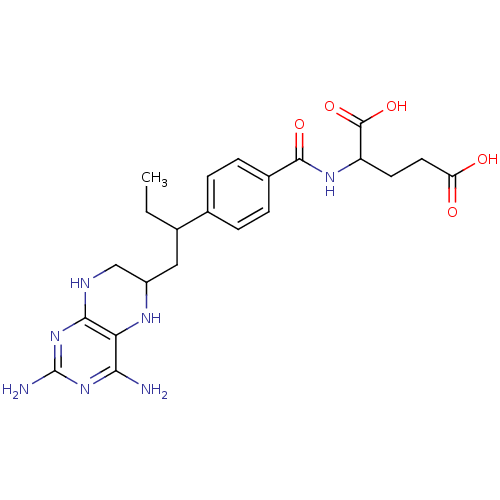 (2-{4-[2-(2,4-Diamino-5,6,7,8-tetrahydro-pteridin-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dihydrofolate reductase (DHFR) derived from Chicken liver | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Gallus gallus (Chicken)) | BDBM50025007 (2-{4-[2-(2,4-Diamino-7,8-dihydro-pteridin-6-yl)-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dihydrofolate reductase (DHFR) derived from Chicken liver | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50405399 (CHEMBL2051989) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dihydrofolate reductase (DHFR) derived from Lactobacillus casei ATCC 7469 | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50405399 (CHEMBL2051989) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dihydrofolate reductase (DHFR) derived from Lactobacillus casei ATCC 7469 | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50405401 (CHEMBL2051990) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dihydrofolate reductase (DHFR) derived from Lactobacillus casei ATCC 7469 | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Gallus gallus (Chicken)) | BDBM50405398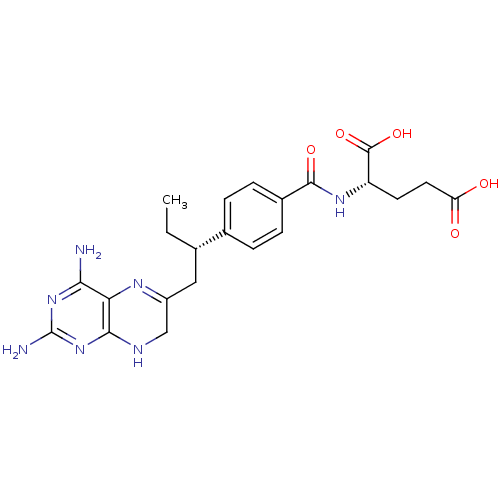 (CHEMBL2051991) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dihydrofolate reductase (DHFR) derived from Chicken liver | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50025008 (2-{4-[2-(2,4-Diamino-5,6,7,8-tetrahydro-pteridin-6...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dihydrofolate reductase (DHFR) derived from Lactobacillus casei ATCC 7469 | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Gallus gallus (Chicken)) | BDBM50405398 (CHEMBL2051991) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dihydrofolate reductase (DHFR) derived from Chicken liver | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50405398 (CHEMBL2051991) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dihydrofolate reductase (DHFR) derived from Lactobacillus casei ATCC 7469 | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50405398 (CHEMBL2051991) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dihydrofolate reductase (DHFR) derived from Lactobacillus casei ATCC 7469 | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||