

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance-P receptor (Homo sapiens (Human)) | BDBM50002660 (((2S,3S)-2-Benzhydryl-1-aza-bicyclo[2.2.2]oct-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0850 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029878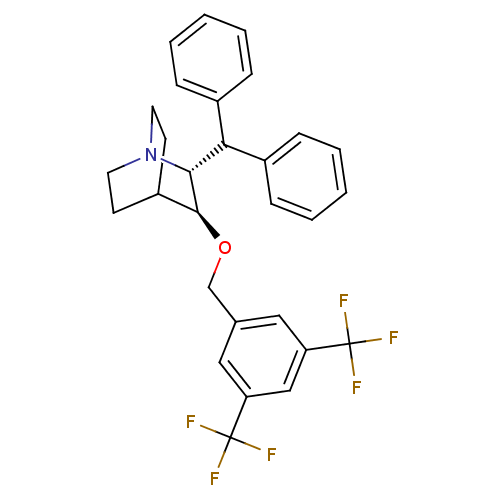 ((2R,3S)-2-Benzhydryl-3-(3,5-bis-trifluoromethyl-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029884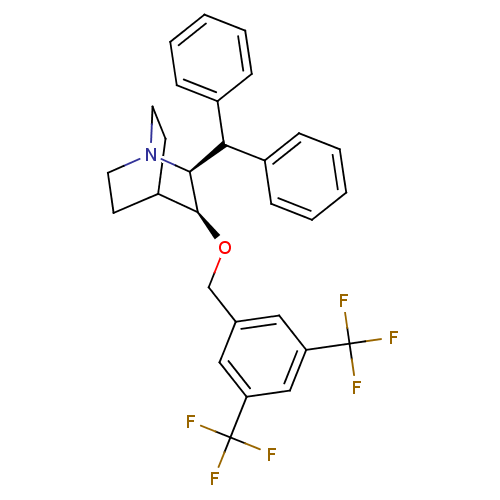 ((2S,3S)-2-Benzhydryl-3-(3,5-bis-trifluoromethyl-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029847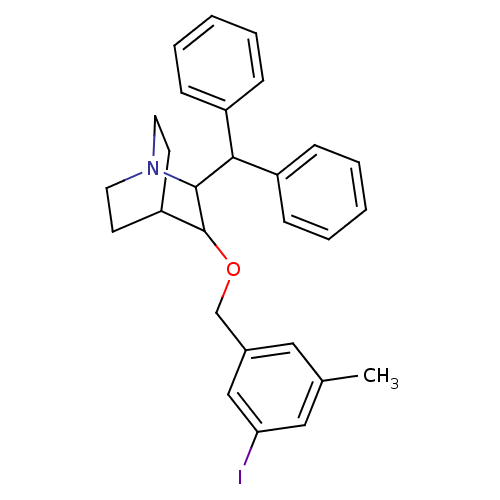 (2-Benzhydryl-3-(3-iodo-5-methyl-benzyloxy)-1-aza-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50000040 (((2S,3S)-2-Benzhydryl-1-aza-bicyclo[2.2.2]oct-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029871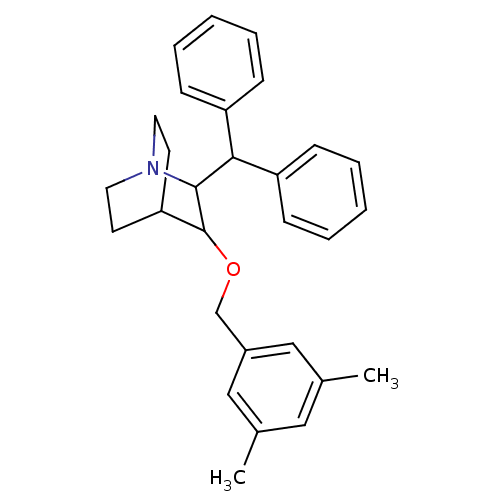 (2-Benzhydryl-3-(3,5-dimethyl-benzyloxy)-1-aza-bicy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029869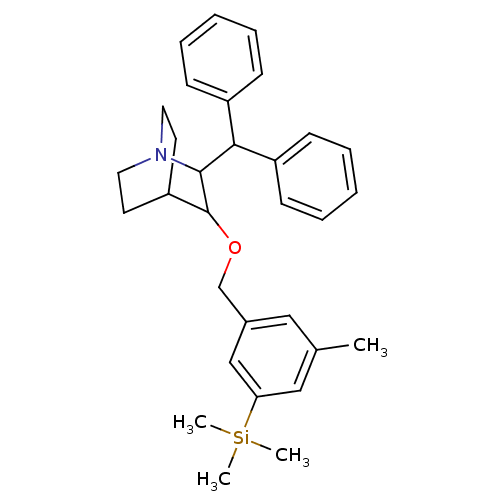 (2-Benzhydryl-3-(3-methyl-5-trimethylsilanyl-benzyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029871 (2-Benzhydryl-3-(3,5-dimethyl-benzyloxy)-1-aza-bicy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029879 (2-Benzhydryl-3-(3-methoxy-5-methyl-benzyloxy)-1-az...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029879 (2-Benzhydryl-3-(3-methoxy-5-methyl-benzyloxy)-1-az...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029839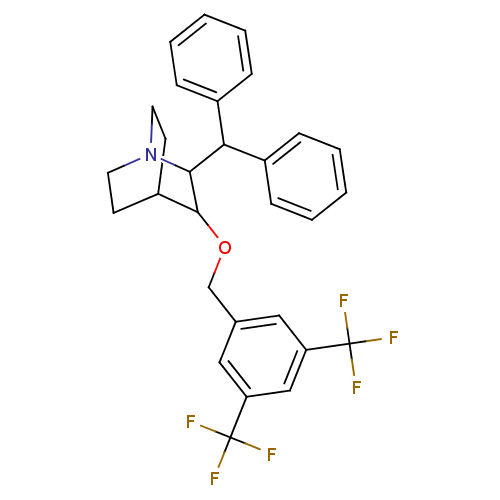 (2-Benzhydryl-3-(3,5-bis-trifluoromethyl-benzyloxy)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029839 (2-Benzhydryl-3-(3,5-bis-trifluoromethyl-benzyloxy)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029850 (3-(3,5-Bis-trifluoromethyl-benzyloxy)-2-(1,2-diphe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029876 (3-(3,5-Bis-trifluoromethyl-benzyloxy)-2-(cyclohexy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029875 (2-Benzhydryl-3-(3,5-dimethoxy-benzyloxy)-1-aza-bic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029875 (2-Benzhydryl-3-(3,5-dimethoxy-benzyloxy)-1-aza-bic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029845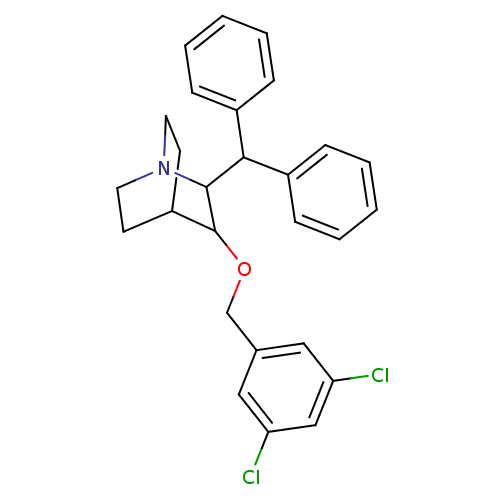 (2-Benzhydryl-3-(3,5-dichloro-benzyloxy)-1-aza-bicy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029845 (2-Benzhydryl-3-(3,5-dichloro-benzyloxy)-1-aza-bicy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029872 (2-Benzhydryl-3-(3-methyl-benzyloxy)-1-aza-bicyclo[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029842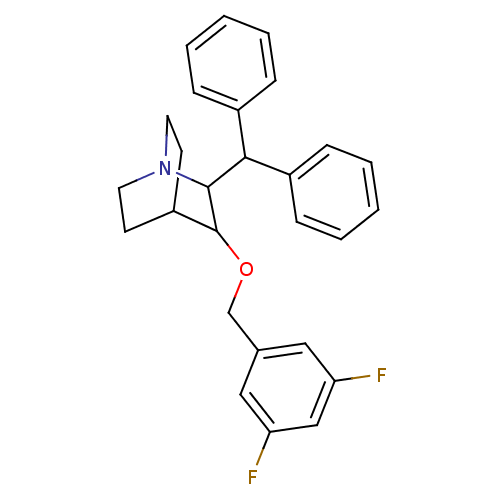 (2-Benzhydryl-3-(3,5-difluoro-benzyloxy)-1-aza-bicy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029863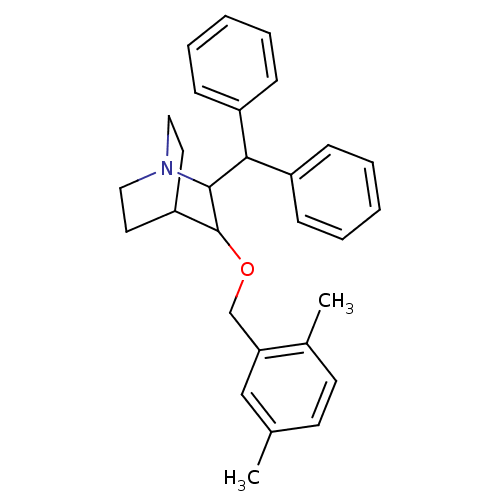 (2-Benzhydryl-3-(2,5-dimethyl-benzyloxy)-1-aza-bicy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029857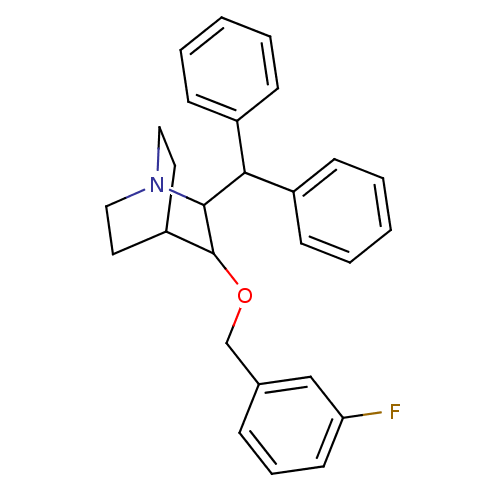 (2-Benzhydryl-3-(3-fluoro-benzyloxy)-1-aza-bicyclo[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029880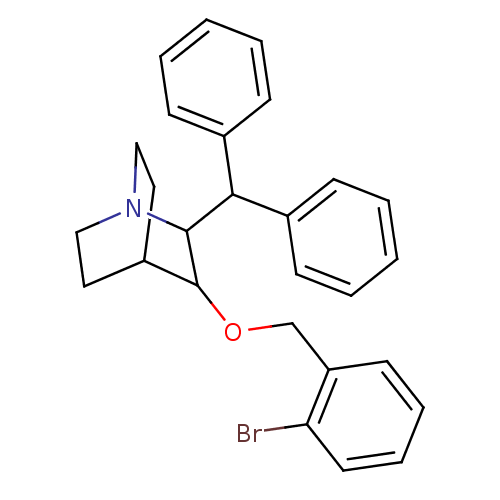 (2-Benzhydryl-3-(2-bromo-benzyloxy)-1-aza-bicyclo[2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029874 (2-Benzhydryl-3-(3-methoxy-benzyloxy)-1-aza-bicyclo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029882 (2-Benzhydryl-3-(3-phenoxy-benzyloxy)-1-aza-bicyclo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029846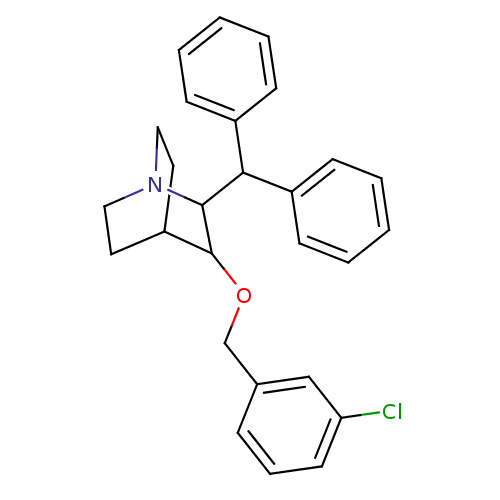 (2-Benzhydryl-3-(3-chloro-benzyloxy)-1-aza-bicyclo[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029877 (2-Benzhydryl-3-(2-trifluoromethyl-benzyloxy)-1-aza...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029860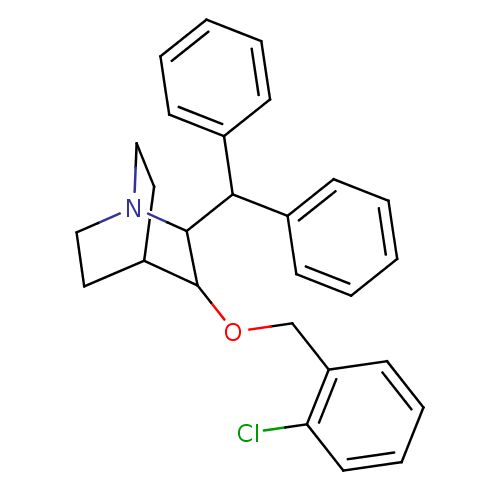 (2-Benzhydryl-3-(2-chloro-benzyloxy)-1-aza-bicyclo[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029887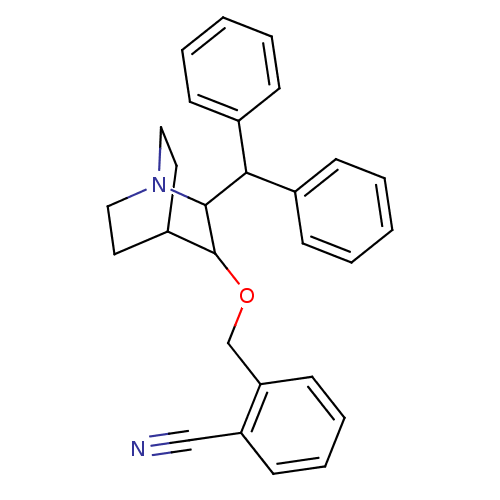 (2-(2-Benzhydryl-1-aza-bicyclo[2.2.2]oct-3-yloxymet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029856 (2-Benzhydryl-3-(2-fluoro-benzyloxy)-1-aza-bicyclo[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029891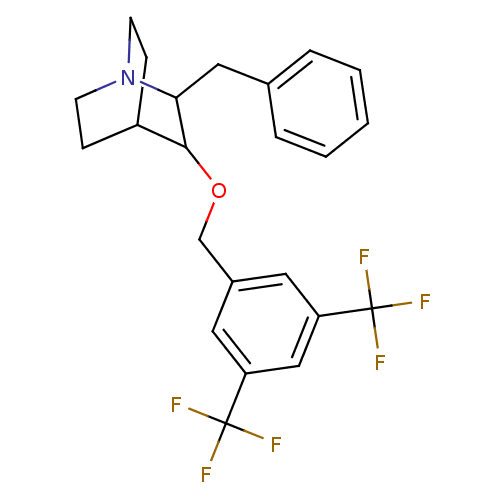 (2-Benzyl-3-(3,5-bis-trifluoromethyl-benzyloxy)-1-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029870 (2-Benzhydryl-3-(2,5-difluoro-benzyloxy)-1-aza-bicy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 106 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029865 (2-Benzyl-3-(3,5-dimethyl-benzyloxy)-1-aza-bicyclo[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029864 (2-Benzhydryl-3-benzyloxy-1-aza-bicyclo[2.2.2]octan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50284614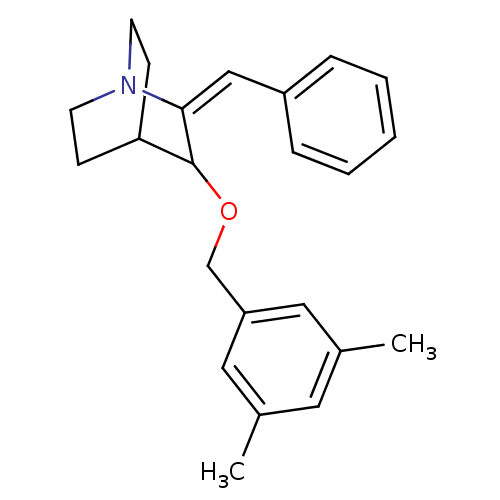 (2-Benzylidene-3-(3,5-dimethyl-benzyloxy)-1-aza-bic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029855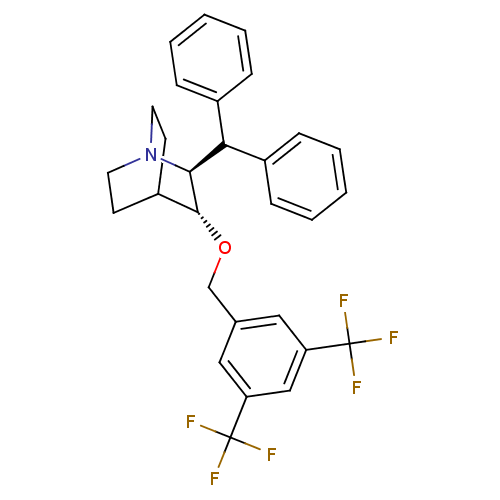 ((2S,3R)-2-Benzhydryl-3-(3,5-bis-trifluoromethyl-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029883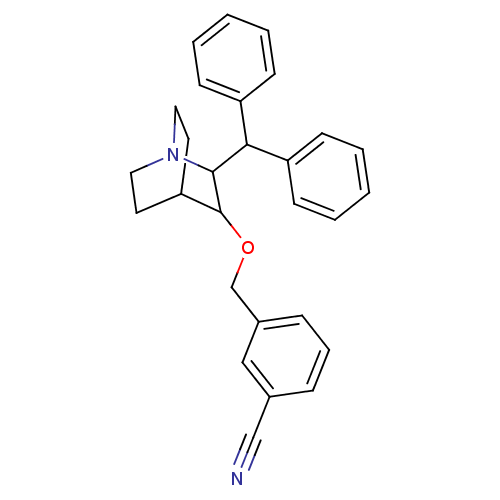 (3-(2-Benzhydryl-1-aza-bicyclo[2.2.2]oct-3-yloxymet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029853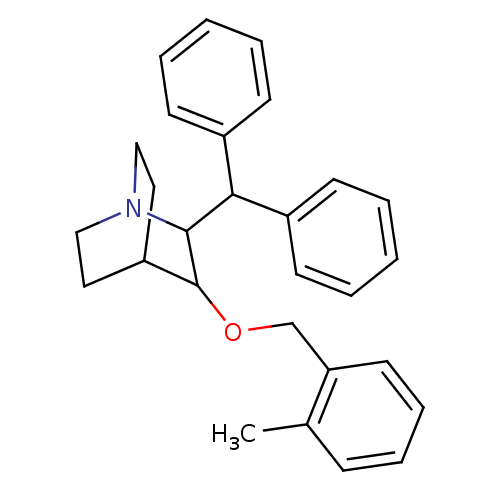 (2-Benzhydryl-3-(2-methyl-benzyloxy)-1-aza-bicyclo[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029854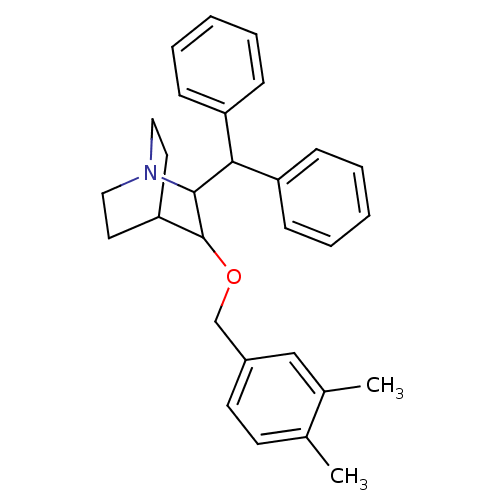 (2-Benzhydryl-3-(3,4-dimethyl-benzyloxy)-1-aza-bicy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 172 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029873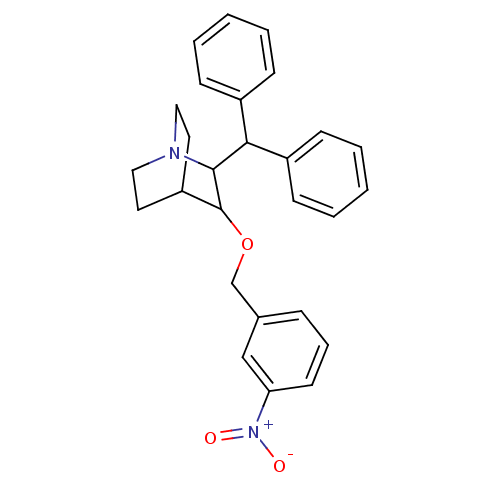 (2-Benzhydryl-3-(3-nitro-benzyloxy)-1-aza-bicyclo[2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 197 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029844 (2-Benzhydryl-3-(4-methyl-benzyloxy)-1-aza-bicyclo[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 246 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029867 (2-Benzhydryl-3-(4-fluoro-benzyloxy)-1-aza-bicyclo[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 252 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029840 (2-Benzhydryl-3-(3-trifluoromethyl-benzyloxy)-1-aza...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029843 ((2R,3R)-2-Benzhydryl-3-(3,5-bis-trifluoromethyl-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029852 (3-(3,5-Dimethyl-benzyloxy)-2-(1,2-diphenyl-ethyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 353 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029852 (3-(3,5-Dimethyl-benzyloxy)-2-(1,2-diphenyl-ethyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 353 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029866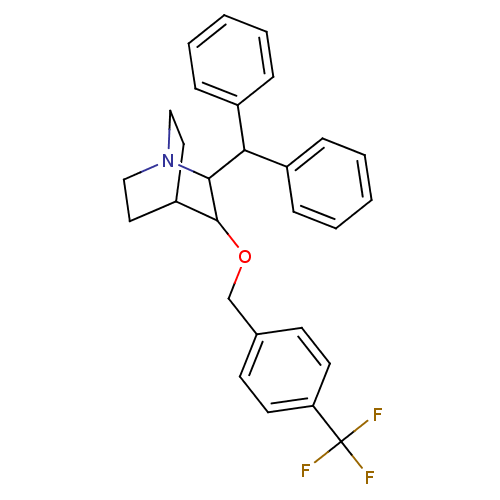 (2-Benzhydryl-3-(4-trifluoromethyl-benzyloxy)-1-aza...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029851 (2-Benzhydryl-3-(4-chloro-benzyloxy)-1-aza-bicyclo[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 417 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029850 (3-(3,5-Bis-trifluoromethyl-benzyloxy)-2-(1,2-diphe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 425 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029881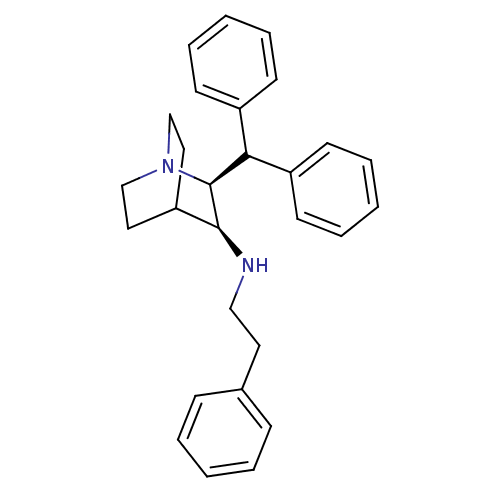 (((2S,3S)-2-Benzhydryl-1-aza-bicyclo[2.2.2]oct-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029849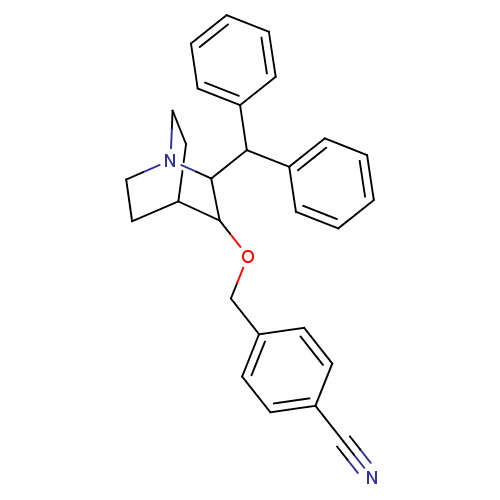 (4-(2-Benzhydryl-1-aza-bicyclo[2.2.2]oct-3-yloxymet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029858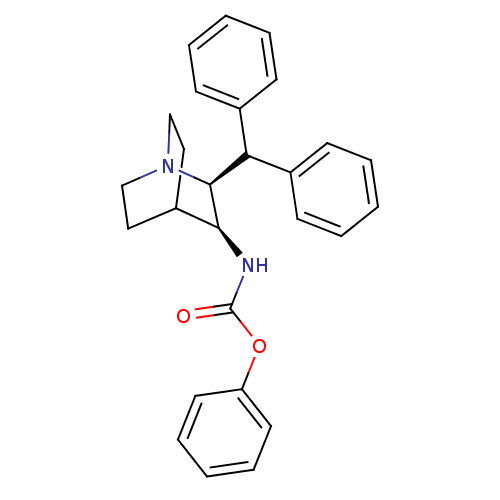 (((2S,3S)-2-Benzhydryl-1-aza-bicyclo[2.2.2]oct-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029848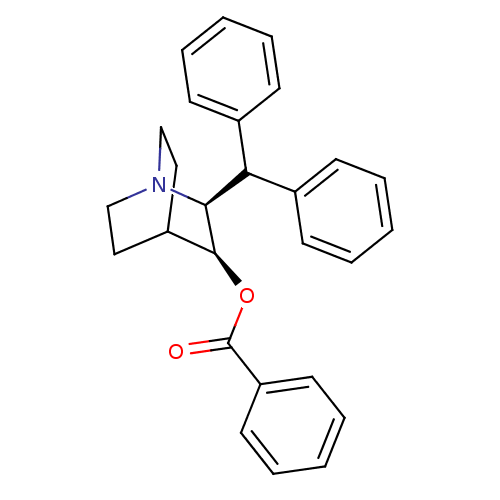 (Benzoic acid (2S,3S)-2-benzhydryl-1-aza-bicyclo[2....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029889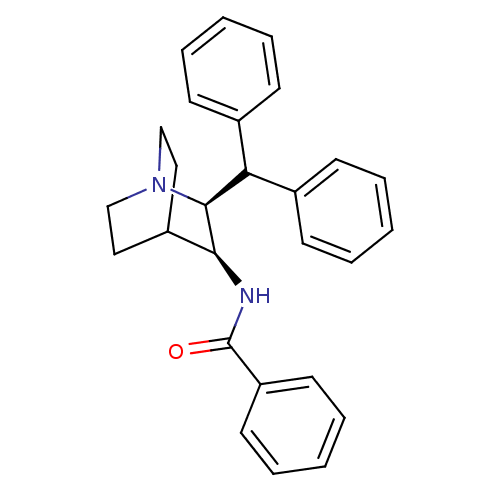 (CHEMBL281502 | N-((2S,3S)-2-Benzhydryl-1-aza-bicyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029888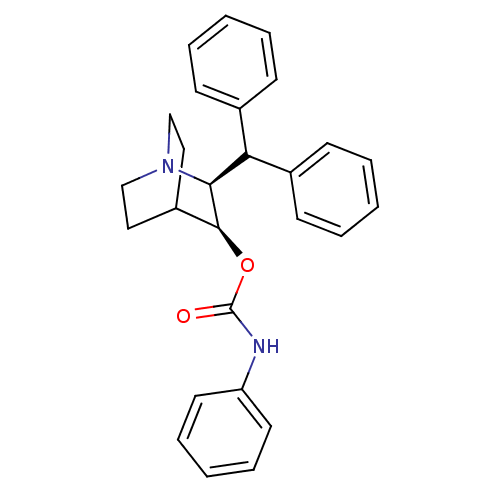 (CHEMBL29760 | Phenyl-carbamic acid (2S,3S)-2-benzh...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029868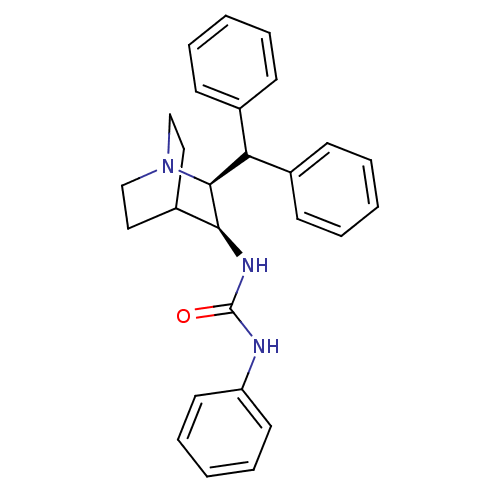 (1-((2S,3S)-2-Benzhydryl-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029890 (CHEMBL281703 | N-((2S,3S)-2-Benzhydryl-1-aza-bicyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029861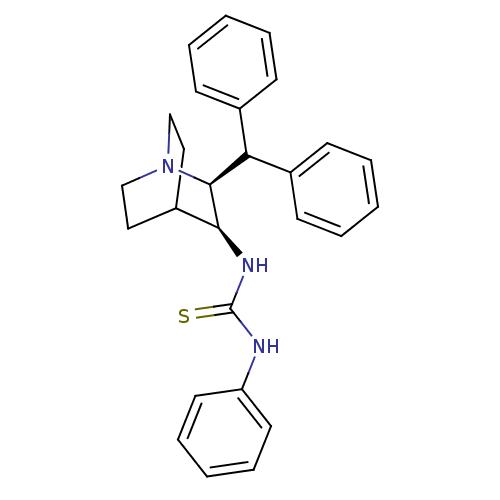 (1-((2S,3S)-2-Benzhydryl-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029886 (2-Benzhydryl-3-(4-methoxy-benzyloxy)-1-aza-bicyclo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50029862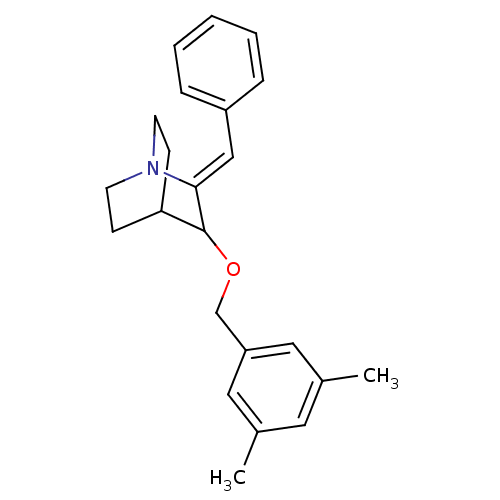 (2-Benzylidene-3-(3,5-dimethyl-benzyloxy)-1-aza-bic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-SP to the CHO cell line expressing human Tachykinin receptor 1 | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50029885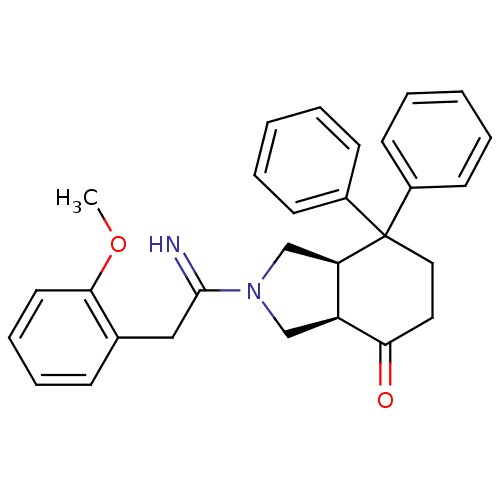 ((3aR,7aR)-2-[1-Imino-2-(2-methoxy-phenyl)-ethyl]-7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 4.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]-SP binding in rat brain membranes | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50029859 (1-Ethyl-2-{(E)-3-[1-ethyl-3,3-dimethyl-1,3-dihydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]-SP binding in rat brain membranes | J Med Chem 38: 4793-805 (1996) BindingDB Entry DOI: 10.7270/Q2PG1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||