

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1018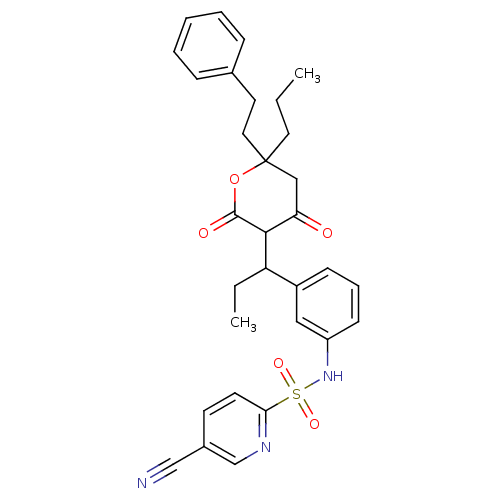 (5-cyano-N-(3-{1-[4-hydroxy-2-oxo-6-(2-phenylethyl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of human immunodeficiency virus type 1 (HIV-1) Protease | Bioorg Med Chem Lett 8: 1237-42 (1999) BindingDB Entry DOI: 10.7270/Q2K64H7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50070300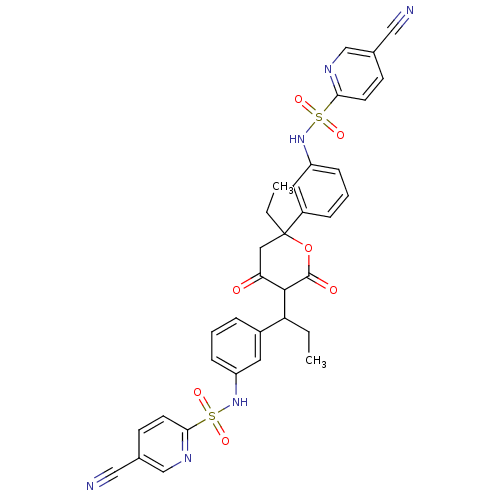 (2N-[3-(1-{2-[3-(5-cyano-2-pyridylsulfonamido)pheny...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of human immunodeficiency virus type 1 (HIV-1) Protease | Bioorg Med Chem Lett 8: 1237-42 (1999) BindingDB Entry DOI: 10.7270/Q2K64H7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM767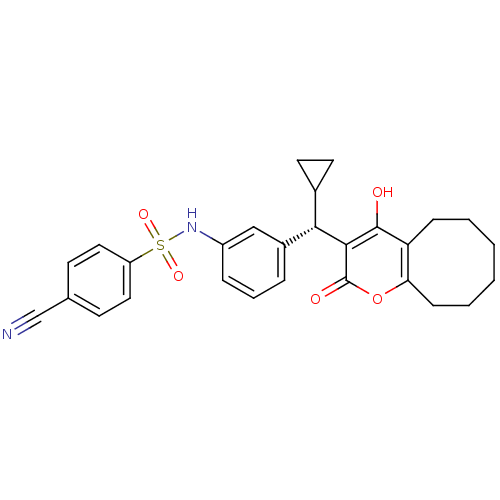 (4-Cyano-N-[3-[cyclopropyl(5,6,7,8,9,10-hexahydro-4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of human immunodeficiency virus type 1 (HIV-1) Protease | Bioorg Med Chem Lett 8: 1237-42 (1999) BindingDB Entry DOI: 10.7270/Q2K64H7T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM548 (CHEMBL21188 | N-(3-{1-[4-hydroxy-2-oxo-6-(2-phenyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of human immunodeficiency virus type 1 (HIV-1) Protease | Bioorg Med Chem Lett 8: 1237-42 (1999) BindingDB Entry DOI: 10.7270/Q2K64H7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM547 (CHEMBL20846 | N-(3-{1-[4-hydroxy-2-oxo-6-(2-phenyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of human immunodeficiency virus type 1 (HIV-1) Protease | Bioorg Med Chem Lett 8: 1237-42 (1999) BindingDB Entry DOI: 10.7270/Q2K64H7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50070299 (4N-[3-(1-{2-ethyl-4-hydroxy-2-[3-(1-methyl-1H-4-im...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of human immunodeficiency virus type 1 (HIV-1) Protease | Bioorg Med Chem Lett 8: 1237-42 (1999) BindingDB Entry DOI: 10.7270/Q2K64H7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM545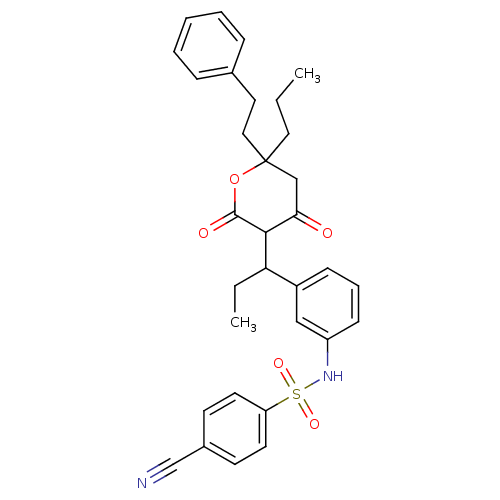 (4-cyano-N-(3-{1-[4-hydroxy-2-oxo-6-(2-phenylethyl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of human immunodeficiency virus type 1 (HIV-1) Protease | Bioorg Med Chem Lett 8: 1237-42 (1999) BindingDB Entry DOI: 10.7270/Q2K64H7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM770 (4-hydroxy-6-(1-phenylbutan-2-yl)-3-(1-phenylpropyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of human immunodeficiency virus type 1 (HIV-1) Protease | Bioorg Med Chem Lett 8: 1237-42 (1999) BindingDB Entry DOI: 10.7270/Q2K64H7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM548 (CHEMBL21188 | N-(3-{1-[4-hydroxy-2-oxo-6-(2-phenyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of human immunodeficiency virus type 1 (HIV-1) Protease | Bioorg Med Chem Lett 8: 1237-42 (1999) BindingDB Entry DOI: 10.7270/Q2K64H7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM767 (4-Cyano-N-[3-[cyclopropyl(5,6,7,8,9,10-hexahydro-4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of human immunodeficiency virus type 1 (HIV-1) Protease | Bioorg Med Chem Lett 8: 1237-42 (1999) BindingDB Entry DOI: 10.7270/Q2K64H7T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM770 (4-hydroxy-6-(1-phenylbutan-2-yl)-3-(1-phenylpropyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of human immunodeficiency virus type 1 (HIV-1) Protease | Bioorg Med Chem Lett 8: 1237-42 (1999) BindingDB Entry DOI: 10.7270/Q2K64H7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||