

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor XI (Homo sapiens (Human)) | BDBM303815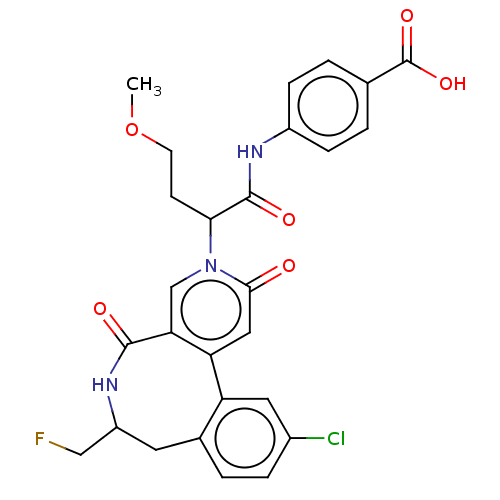 (4-({2-[11-Chloro-7-(fluoromethyl)-2,5-dioxo-5,6,7,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM303810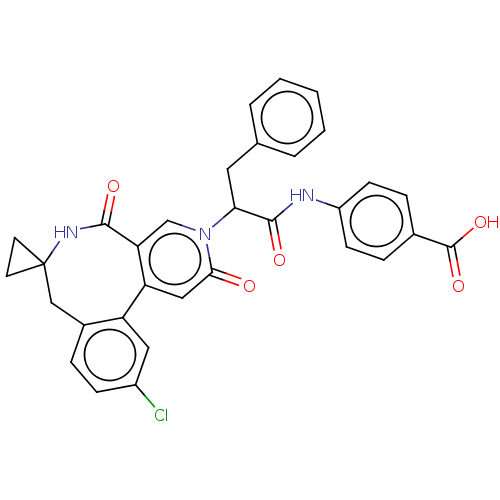 (4-{[2-(11′-Chloro-2′,5′-dioxo-2&...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM303794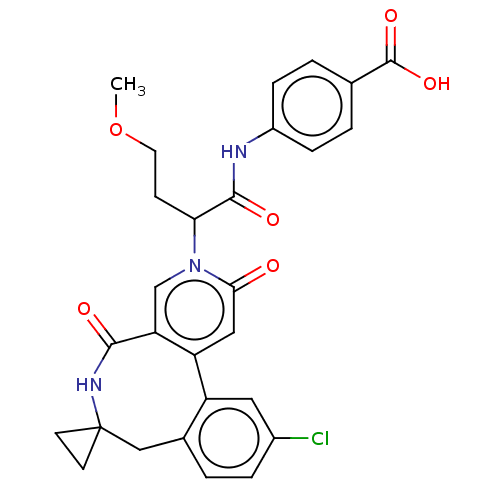 (4-{[2-(11′-Chloro-2′,5′-dioxo-2&...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM303807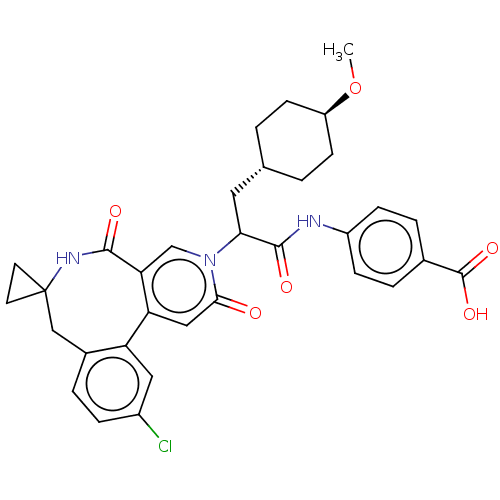 (4-{[2-(11′-Chloro-2′,5′-dioxo-2&...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM303813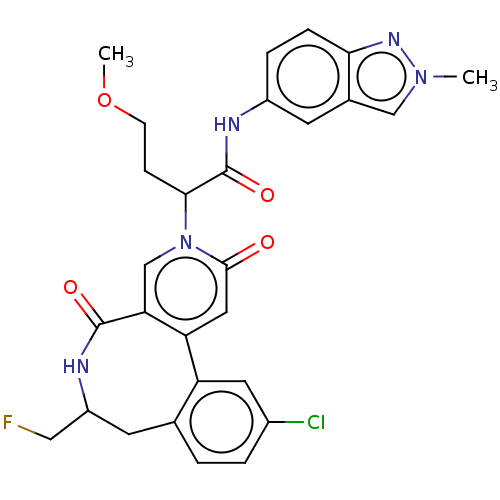 (2-[11-Chloro-7-(fluoromethyl)-2,5-dioxo-5,6,7,8-te...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM303804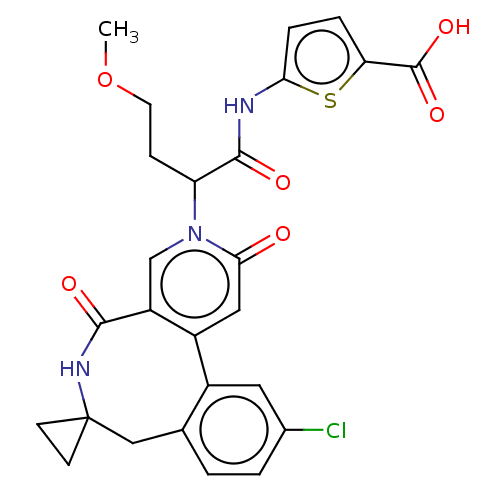 (5-{[2-(11′-Chloro-2′,5′-dioxo-2&...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM303812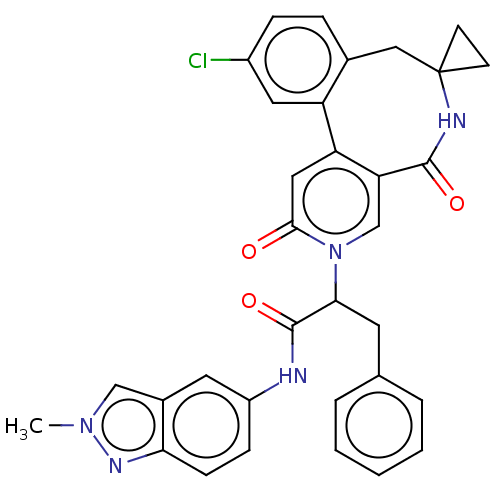 (2-(11′-Chloro-2′,5′-dioxo-2̸...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM303811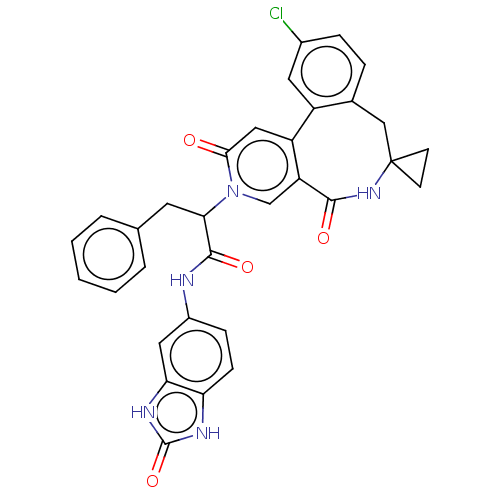 (2-(11′-Chloro-2′,5′-dioxo-2̸...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM303814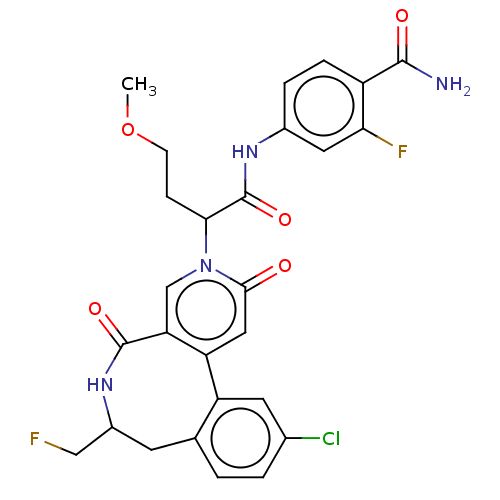 (4-({2-[11-Chloro-7-(fluoromethyl)-2,5-dioxo-5,6,7,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM303792 (4-{[2-(11′-Chloro-2′,5′-dioxo-2&...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM303797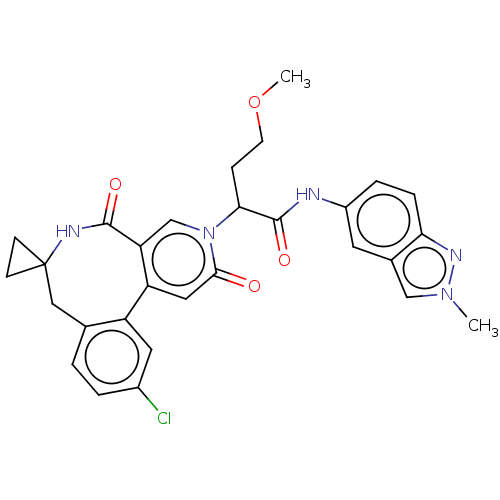 (2-(11′-Chloro-2′,5′-dioxo-2̸...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM303801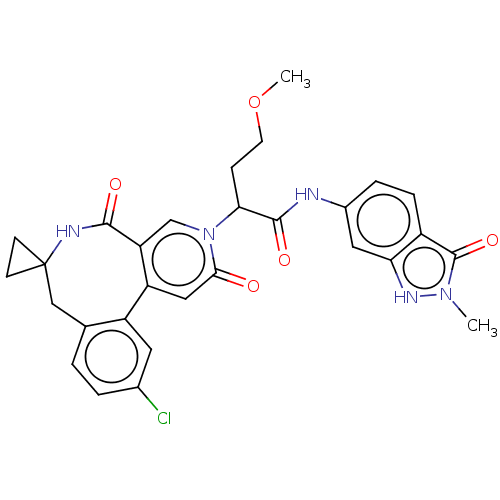 (2-(11′-Chloro-2′,5′-dioxo-2̸...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM303798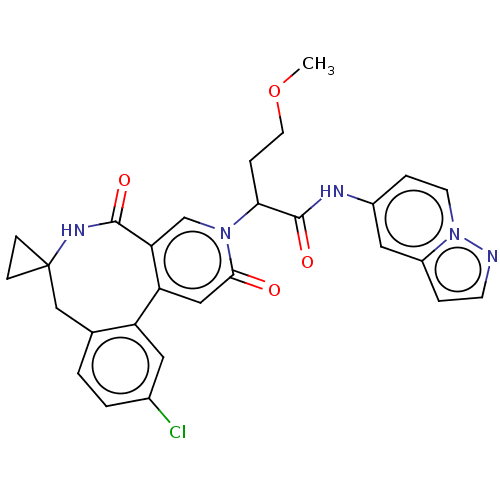 (2-(11′-Chloro-2′,5′-dioxo-2̸...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM303793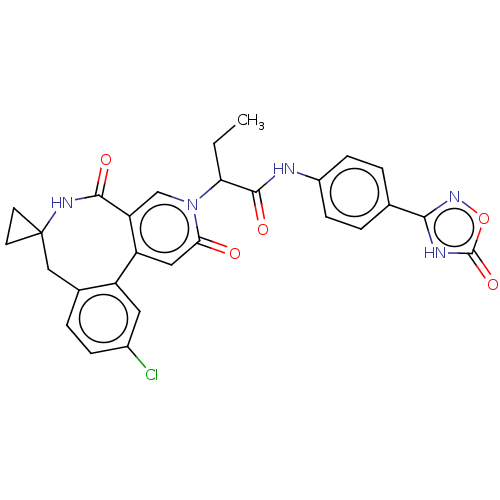 (2-(11′-Chloro-2′,5′-dioxo-2̸...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM303796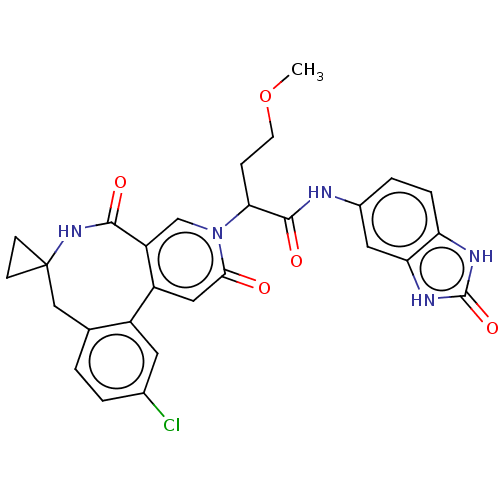 (2-(11′-Chloro-2′,5′-dioxo-2̸...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM303809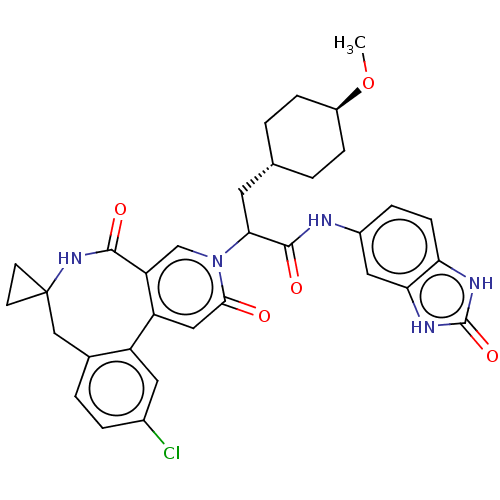 (2-(11′-Chloro-2′,5′-dioxo-2̸...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM303800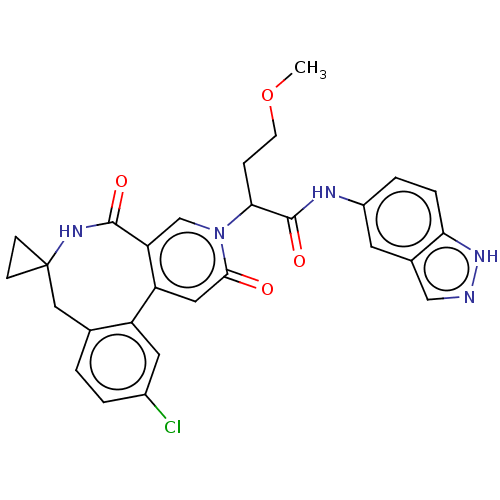 (2-(11′-Chloro-2′,5′-dioxo-2̸...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM303795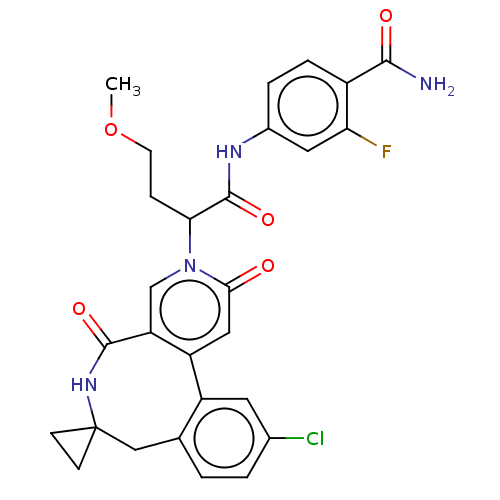 (4-{[2-(11′-Chloro-2′,5′-dioxo-2&...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM303788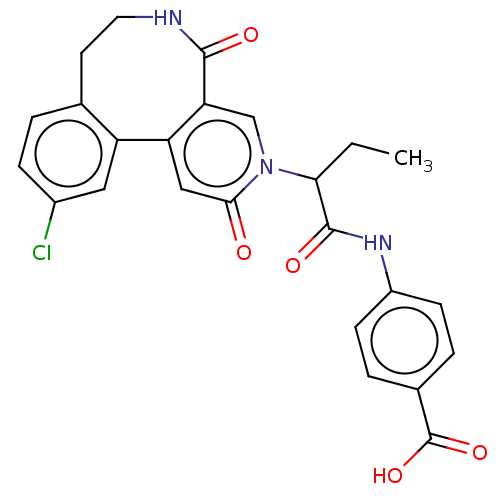 (4-{[2-(11-Chloro-2,5-dioxo-5,6,7,8-tetrahydropyrid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM303808 (4-{[2-(11′-Chloro-2′,5′-dioxo-2&...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM303803 (5-{[2-(11′-Chloro-2′,5′-dioxo-2&...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM303806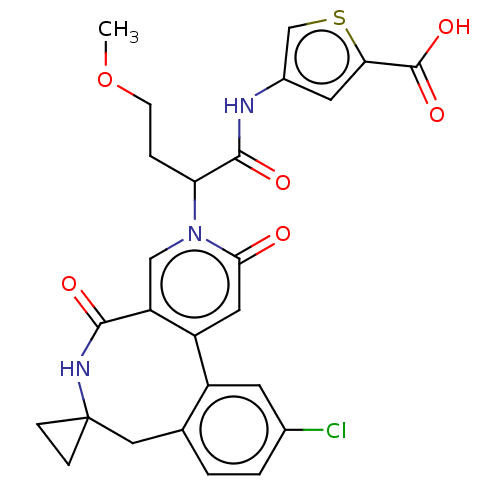 (4-{[2-(11′-Chloro-2′,5′-dioxo-2&...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM303790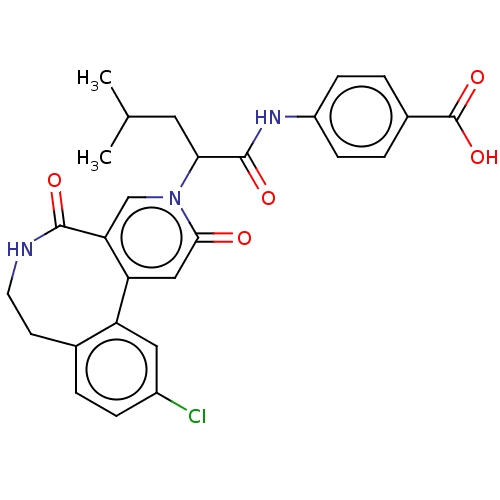 (4-{[2-(11-Chloro-2,5-dioxo-5,6,7,8-tetrahydropyrid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM303813 (2-[11-Chloro-7-(fluoromethyl)-2,5-dioxo-5,6,7,8-te...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description o determine the plasma kallikrein inhibition of the substances according to the invention, a biochemical test system is used which utilizes the react... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM303811 (2-(11′-Chloro-2′,5′-dioxo-2̸...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description o determine the plasma kallikrein inhibition of the substances according to the invention, a biochemical test system is used which utilizes the react... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM303799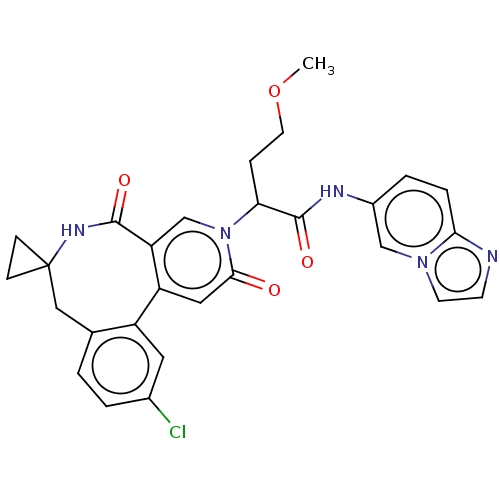 (2-(11′-Chloro-2′,5′-dioxo-2̸...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM303789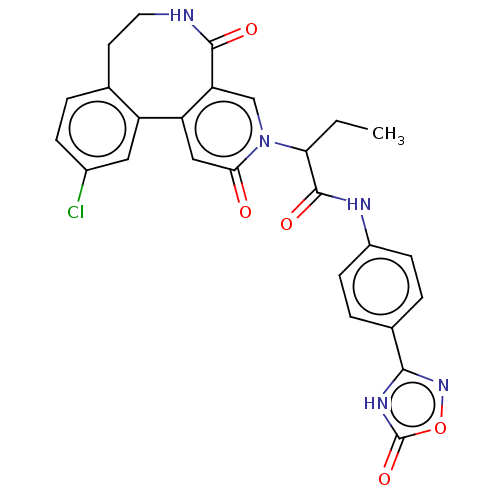 (2-(11-Chloro-2,5-dioxo-5,6,7,8-tetrahydropyrido[3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM303812 (2-(11′-Chloro-2′,5′-dioxo-2̸...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description o determine the plasma kallikrein inhibition of the substances according to the invention, a biochemical test system is used which utilizes the react... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM303802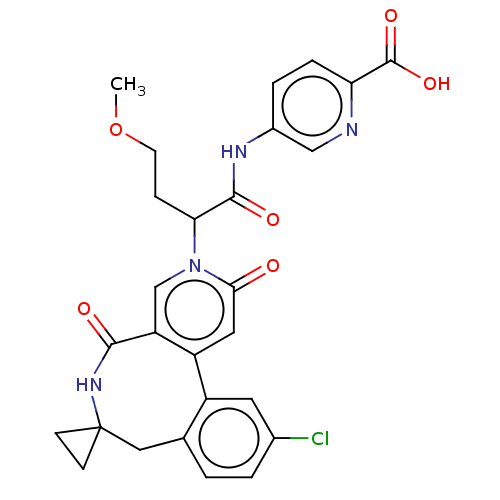 (5-{[2-(11′-Chloro-2′,5′-dioxo-2&...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM303810 (4-{[2-(11′-Chloro-2′,5′-dioxo-2&...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description o determine the plasma kallikrein inhibition of the substances according to the invention, a biochemical test system is used which utilizes the react... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM303815 (4-({2-[11-Chloro-7-(fluoromethyl)-2,5-dioxo-5,6,7,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description o determine the plasma kallikrein inhibition of the substances according to the invention, a biochemical test system is used which utilizes the react... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM303805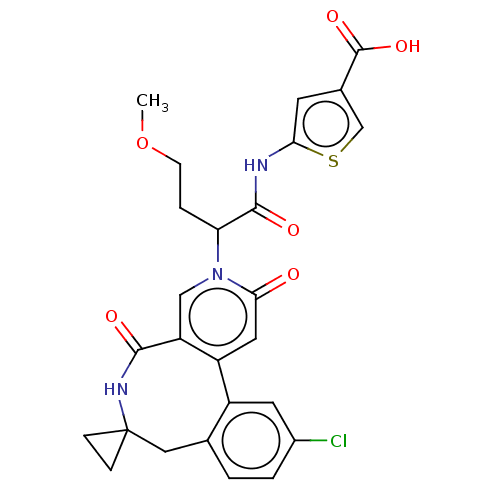 (5-{[2-(11′-Chloro-2′,5′-dioxo-2&...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM303814 (4-({2-[11-Chloro-7-(fluoromethyl)-2,5-dioxo-5,6,7,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description o determine the plasma kallikrein inhibition of the substances according to the invention, a biochemical test system is used which utilizes the react... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM303791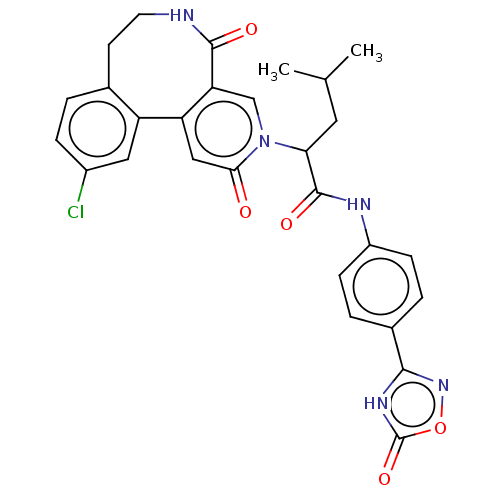 (2-(11-Chloro-2,5-dioxo-5,6,7,8-tetrahydropyrido[3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM303785 (4-{[2-(10′-Chloro-2′,5′-dioxo-5&...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM303798 (2-(11′-Chloro-2′,5′-dioxo-2̸...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description o determine the plasma kallikrein inhibition of the substances according to the invention, a biochemical test system is used which utilizes the react... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM303801 (2-(11′-Chloro-2′,5′-dioxo-2̸...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description o determine the plasma kallikrein inhibition of the substances according to the invention, a biochemical test system is used which utilizes the react... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM303796 (2-(11′-Chloro-2′,5′-dioxo-2̸...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description o determine the plasma kallikrein inhibition of the substances according to the invention, a biochemical test system is used which utilizes the react... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM303797 (2-(11′-Chloro-2′,5′-dioxo-2̸...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description o determine the plasma kallikrein inhibition of the substances according to the invention, a biochemical test system is used which utilizes the react... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM303809 (2-(11′-Chloro-2′,5′-dioxo-2̸...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description o determine the plasma kallikrein inhibition of the substances according to the invention, a biochemical test system is used which utilizes the react... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM303784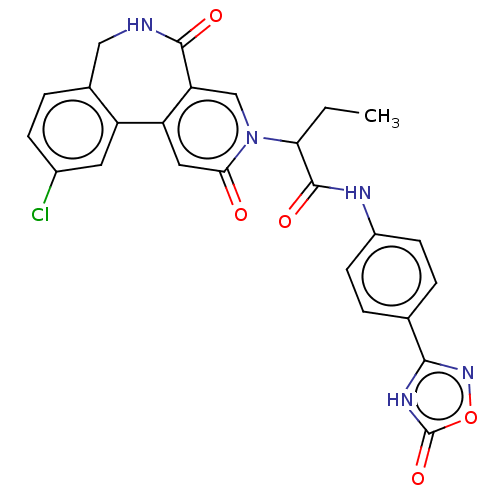 (2-(10-Chloro-2,5-dioxo-2,5,6,7-tetrahydro-3H-pyrid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM303800 (2-(11′-Chloro-2′,5′-dioxo-2̸...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description o determine the plasma kallikrein inhibition of the substances according to the invention, a biochemical test system is used which utilizes the react... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM303804 (5-{[2-(11′-Chloro-2′,5′-dioxo-2&...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description o determine the plasma kallikrein inhibition of the substances according to the invention, a biochemical test system is used which utilizes the react... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM303787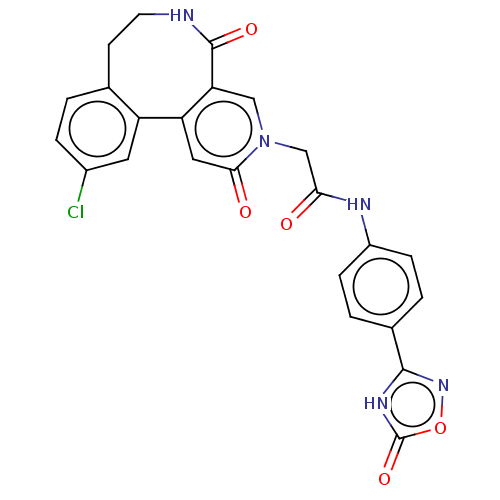 (2-(11-Chloro-2,5-dioxo-5,6,7,8-tetrahydropyrido[3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM303807 (4-{[2-(11′-Chloro-2′,5′-dioxo-2&...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description o determine the plasma kallikrein inhibition of the substances according to the invention, a biochemical test system is used which utilizes the react... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM303794 (4-{[2-(11′-Chloro-2′,5′-dioxo-2&...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description o determine the plasma kallikrein inhibition of the substances according to the invention, a biochemical test system is used which utilizes the react... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM303783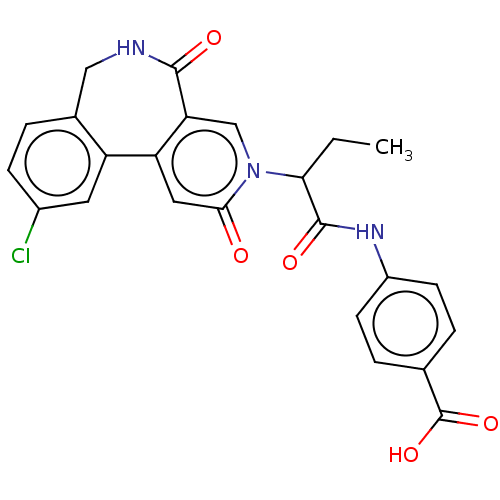 (4-{[2-(10-Chloro-2,5-dioxo-2,5,6,7-tetrahydro-3H-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM303790 (4-{[2-(11-Chloro-2,5-dioxo-5,6,7,8-tetrahydropyrid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description o determine the plasma kallikrein inhibition of the substances according to the invention, a biochemical test system is used which utilizes the react... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM303786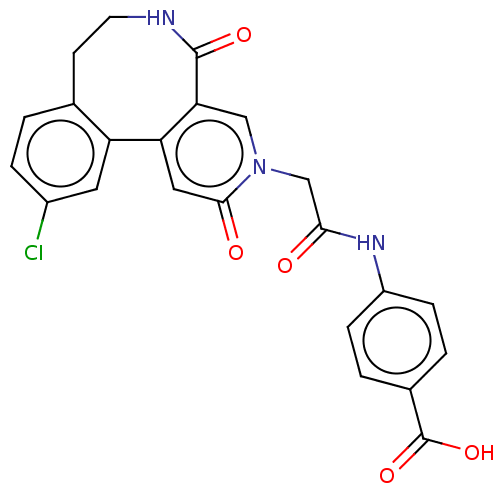 (4-{[(11-Chloro-2,5-dioxo-5,6,7,8-tetrahydropyrido[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM303808 (4-{[2-(11′-Chloro-2′,5′-dioxo-2&...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description o determine the plasma kallikrein inhibition of the substances according to the invention, a biochemical test system is used which utilizes the react... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM303795 (4-{[2-(11′-Chloro-2′,5′-dioxo-2&...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description o determine the plasma kallikrein inhibition of the substances according to the invention, a biochemical test system is used which utilizes the react... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM303806 (4-{[2-(11′-Chloro-2′,5′-dioxo-2&...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description o determine the plasma kallikrein inhibition of the substances according to the invention, a biochemical test system is used which utilizes the react... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM303803 (5-{[2-(11′-Chloro-2′,5′-dioxo-2&...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description o determine the plasma kallikrein inhibition of the substances according to the invention, a biochemical test system is used which utilizes the react... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM303799 (2-(11′-Chloro-2′,5′-dioxo-2̸...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description o determine the plasma kallikrein inhibition of the substances according to the invention, a biochemical test system is used which utilizes the react... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM303793 (2-(11′-Chloro-2′,5′-dioxo-2̸...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description o determine the plasma kallikrein inhibition of the substances according to the invention, a biochemical test system is used which utilizes the react... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM303788 (4-{[2-(11-Chloro-2,5-dioxo-5,6,7,8-tetrahydropyrid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description o determine the plasma kallikrein inhibition of the substances according to the invention, a biochemical test system is used which utilizes the react... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM303792 (4-{[2-(11′-Chloro-2′,5′-dioxo-2&...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description o determine the plasma kallikrein inhibition of the substances according to the invention, a biochemical test system is used which utilizes the react... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM303789 (2-(11-Chloro-2,5-dioxo-5,6,7,8-tetrahydropyrido[3,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description o determine the plasma kallikrein inhibition of the substances according to the invention, a biochemical test system is used which utilizes the react... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM303791 (2-(11-Chloro-2,5-dioxo-5,6,7,8-tetrahydropyrido[3,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description o determine the plasma kallikrein inhibition of the substances according to the invention, a biochemical test system is used which utilizes the react... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM303805 (5-{[2-(11′-Chloro-2′,5′-dioxo-2&...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description o determine the plasma kallikrein inhibition of the substances according to the invention, a biochemical test system is used which utilizes the react... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM303802 (5-{[2-(11′-Chloro-2′,5′-dioxo-2&...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description o determine the plasma kallikrein inhibition of the substances according to the invention, a biochemical test system is used which utilizes the react... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM303784 (2-(10-Chloro-2,5-dioxo-2,5,6,7-tetrahydro-3H-pyrid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description o determine the plasma kallikrein inhibition of the substances according to the invention, a biochemical test system is used which utilizes the react... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM303787 (2-(11-Chloro-2,5-dioxo-5,6,7,8-tetrahydropyrido[3,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description o determine the plasma kallikrein inhibition of the substances according to the invention, a biochemical test system is used which utilizes the react... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM303783 (4-{[2-(10-Chloro-2,5-dioxo-2,5,6,7-tetrahydro-3H-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description o determine the plasma kallikrein inhibition of the substances according to the invention, a biochemical test system is used which utilizes the react... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM303786 (4-{[(11-Chloro-2,5-dioxo-5,6,7,8-tetrahydropyrido[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description o determine the plasma kallikrein inhibition of the substances according to the invention, a biochemical test system is used which utilizes the react... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM303785 (4-{[2-(10′-Chloro-2′,5′-dioxo-5&...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description o determine the plasma kallikrein inhibition of the substances according to the invention, a biochemical test system is used which utilizes the react... | US Patent US10138236 (2018) BindingDB Entry DOI: 10.7270/Q2445PH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||