| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Tyrosine-protein phosphatase non-receptor type 1 [1-298] |
|---|
| Ligand | BDBM13469 |
|---|
| Substrate/Competitor | BDBM13466 |
|---|
| Meas. Tech. | Phosphatase Inhibition Assay |
|---|
| pH | 7±n/a |
|---|
| Temperature | 295.15±n/a K |
|---|
| IC50 | 1.7±n/a nM |
|---|
| Citation |  Ala, PJ; Gonneville, L; Hillman, M; Becker-Pasha, M; Yue, EW; Douty, B; Wayland, B; Polam, P; Crawley, ML; McLaughlin, E; Sparks, RB; Glass, B; Takvorian, A; Combs, AP; Burn, TC; Hollis, GF; Wynn, R Structural insights into the design of nonpeptidic isothiazolidinone-containing inhibitors of protein-tyrosine phosphatase 1B. J Biol Chem281:38013-21 (2006) [PubMed] Article Ala, PJ; Gonneville, L; Hillman, M; Becker-Pasha, M; Yue, EW; Douty, B; Wayland, B; Polam, P; Crawley, ML; McLaughlin, E; Sparks, RB; Glass, B; Takvorian, A; Combs, AP; Burn, TC; Hollis, GF; Wynn, R Structural insights into the design of nonpeptidic isothiazolidinone-containing inhibitors of protein-tyrosine phosphatase 1B. J Biol Chem281:38013-21 (2006) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Inhibition_Run data, Solution Info, Assay Method |
|---|
| |
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] |
|---|
| Name: | Tyrosine-protein phosphatase non-receptor type 1 [1-298] |
|---|
| Synonyms: | PTN1_HUMAN | PTP-1B | PTP1B | PTPN1 | PTPase 1B | Protein-Tyrosine Phosphatase 1B (PTP1B) | Tyrosine-protein phosphatase, non-receptor type 1 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 34670.65 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | The catalytic domain of PTP 1B (residues 1-298) was expressed and purified from E. coli. |
|---|
| Residue: | 298 |
|---|
| Sequence: | MEMEKEFEQIDKSGSWAAIYQDIRHEASDFPCRVAKLPKNKNRNRYRDVSPFDHSRIKLH
QEDNDYINASLIKMEEAQRSYILTQGPLPNTCGHFWEMVWEQKSRGVVMLNRVMEKGSLK
CAQYWPQKEEKEMIFEDTNLKLTLISEDIKSYYTVRQLELENLTTQETREILHFHYTTWP
DFGVPESPASFLNFLFKVRESGSLSPEHGPVVVHCSAGIGRSGTFCLADTCLLLMDKRKD
PSSVDIKKVLLEMRKFRMGLIQTADQLRFSYLAVIEGAKFIMGDSSVQDQWKELSHED
|
|
|
|---|
| BDBM13469 |
|---|
| BDBM13466 |
|---|
| Name | BDBM13469 |
|---|
| Synonyms: | ({4-[(2S)-2-carbamoyl-2-[(2S)-2-(1-{4-[difluoro(phosphono)methyl]phenyl}acetamido)-3-phenylpropanamido]ethyl]phenyl}difluoromethyl)phosphonic acid | Difluoromethylphosphonic acid (DFMP) deriv. 1 | [[4-[[(1S)-1-[[(1S)-1-aminocarbonyl-2-[4-(difluoro-phosphono-methyl)phenyl]ethyl]carbamoyl]-2-phenyl-ethyl]carbamoylmethyl]phenyl]-difluoro-methyl]phosphonic acid |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C28H29F4N3O9P2 |
|---|
| Mol. Mass. | 689.4857 |
|---|
| SMILES | NC(=O)[C@H](Cc1ccc(cc1)C(F)(F)P(O)(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)Cc1ccc(cc1)C(F)(F)P(O)(O)=O |r| |
|---|
| Structure | 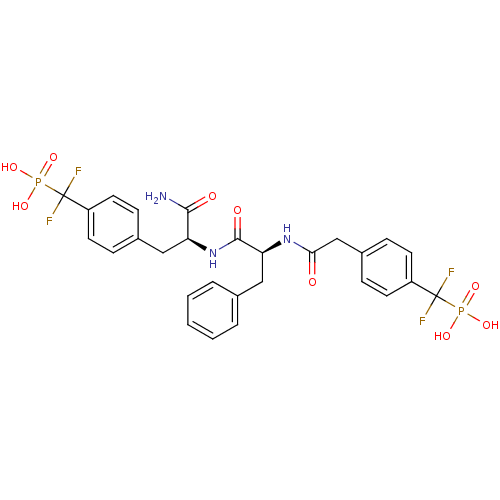
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Ala, PJ; Gonneville, L; Hillman, M; Becker-Pasha, M; Yue, EW; Douty, B; Wayland, B; Polam, P; Crawley, ML; McLaughlin, E; Sparks, RB; Glass, B; Takvorian, A; Combs, AP; Burn, TC; Hollis, GF; Wynn, R Structural insights into the design of nonpeptidic isothiazolidinone-containing inhibitors of protein-tyrosine phosphatase 1B. J Biol Chem281:38013-21 (2006) [PubMed] Article
Ala, PJ; Gonneville, L; Hillman, M; Becker-Pasha, M; Yue, EW; Douty, B; Wayland, B; Polam, P; Crawley, ML; McLaughlin, E; Sparks, RB; Glass, B; Takvorian, A; Combs, AP; Burn, TC; Hollis, GF; Wynn, R Structural insights into the design of nonpeptidic isothiazolidinone-containing inhibitors of protein-tyrosine phosphatase 1B. J Biol Chem281:38013-21 (2006) [PubMed] Article