

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM13465 ((2S)-N-[(1S)-1-carbamoyl-2-{4-[(5S)-1,1,3-trioxo-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition constant against protein-tyrosine phosphatase 1B by PNPP enzyme assay | J Med Chem 48: 6544-8 (2005) Article DOI: 10.1021/jm0504555 BindingDB Entry DOI: 10.7270/Q2805252 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50300312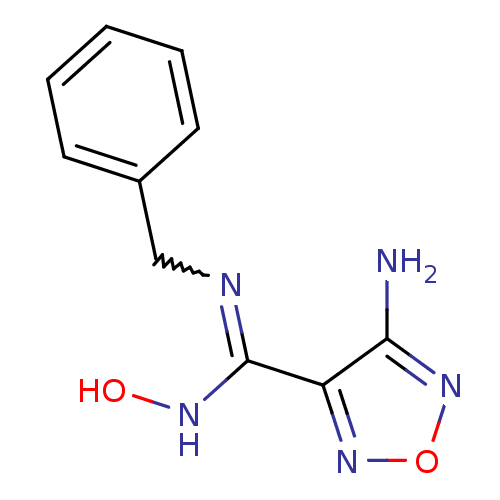 (4-amino-1,2,5-oxadiazole-3-carboximidamide | CHEMB...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of N-terminal his-tagged human indoleamine 2,3-dioxygenase expressed in Escherichia coli assessed as N'-formylkynurenine formation by spec... | J Med Chem 52: 7364-7 (2009) Article DOI: 10.1021/jm900518f BindingDB Entry DOI: 10.7270/Q29P32KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50300305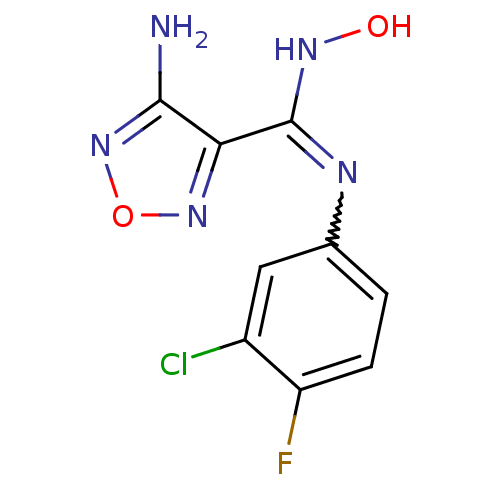 (4-Amino-N-(3-chloro-4-fluorophenyl)-N'-hydroxy-1,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Competitive inhibition of IDO1 (unknown origin) | ACS Med Chem Lett 8: 486-491 (2017) Article DOI: 10.1021/acsmedchemlett.6b00391 BindingDB Entry DOI: 10.7270/Q2G73H0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293912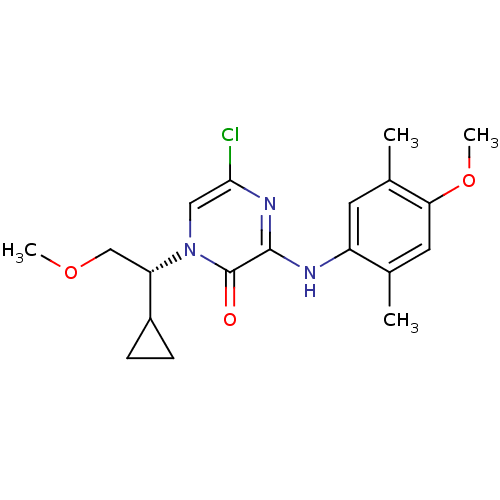 ((R)-5-chloro-1-(1-cyclopropyl-2-methoxyethyl)-3-(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]ovine-CRF from CRF1 receptor in rat frontal cortex by rapid filtration technique | J Med Chem 52: 4161-72 (2009) Article DOI: 10.1021/jm900302q BindingDB Entry DOI: 10.7270/Q2WW7JM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293912 ((R)-5-chloro-1-(1-cyclopropyl-2-methoxyethyl)-3-(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | J Med Chem 52: 4173-91 (2009) Article DOI: 10.1021/jm900301y BindingDB Entry DOI: 10.7270/Q2G44Q9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293974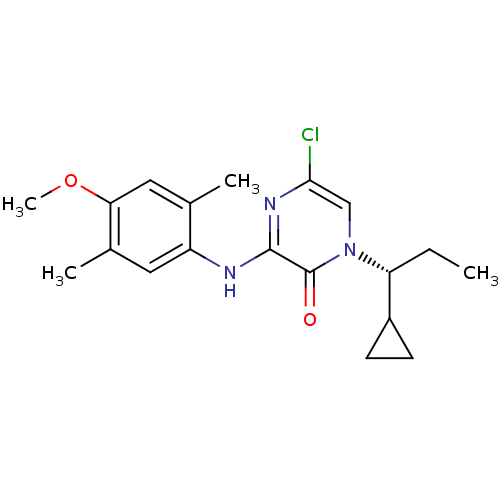 ((R)-5-Chloro-1-(1-cyclopropylpropyl)-3-(4-methoxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | J Med Chem 52: 4173-91 (2009) Article DOI: 10.1021/jm900301y BindingDB Entry DOI: 10.7270/Q2G44Q9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293964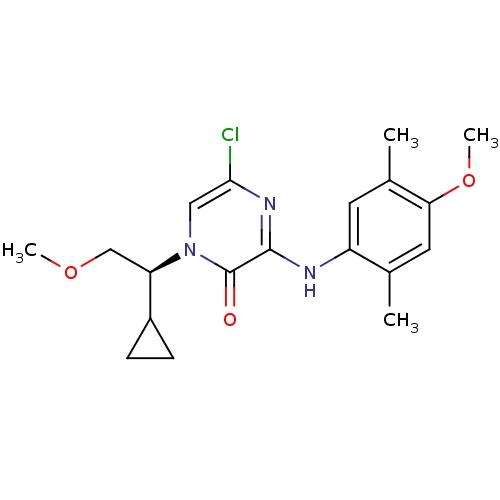 ((S)-5-Chloro-1-(1-cyclopropyl-2-methoxyethyl)-3-(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | J Med Chem 52: 4173-91 (2009) Article DOI: 10.1021/jm900301y BindingDB Entry DOI: 10.7270/Q2G44Q9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13467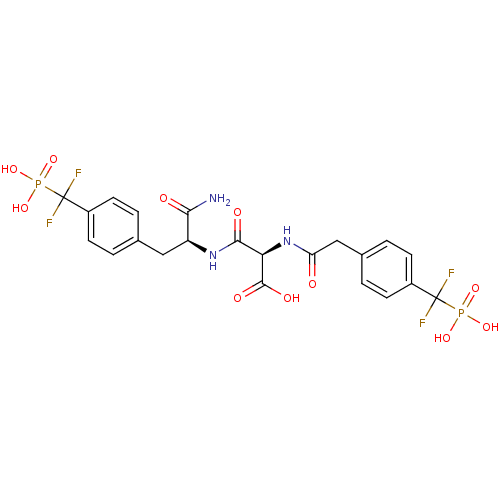 ((2R)-2-{[(1S)-1-carbamoyl-2-{4-[difluoro(phosphono...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 38013-21 (2006) Article DOI: 10.1074/jbc.M607913200 BindingDB Entry DOI: 10.7270/Q2JW8C4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293941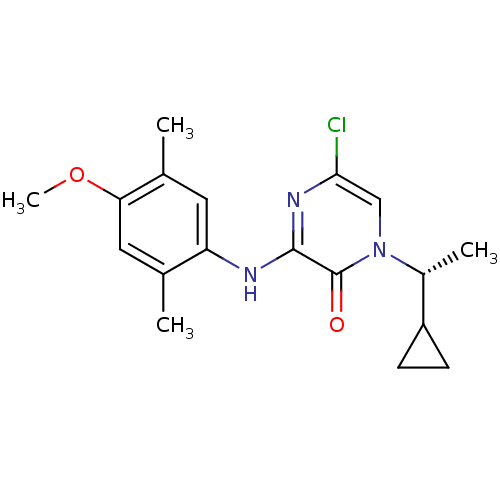 ((R)-5-Chloro-1-(1-cyclopropylethyl)-3-(4-methoxy-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]ovine-CRF from CRF1 receptor in rat frontal cortex by rapid filtration technique | J Med Chem 52: 4161-72 (2009) Article DOI: 10.1021/jm900302q BindingDB Entry DOI: 10.7270/Q2WW7JM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293941 ((R)-5-Chloro-1-(1-cyclopropylethyl)-3-(4-methoxy-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | J Med Chem 52: 4173-91 (2009) Article DOI: 10.1021/jm900301y BindingDB Entry DOI: 10.7270/Q2G44Q9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293916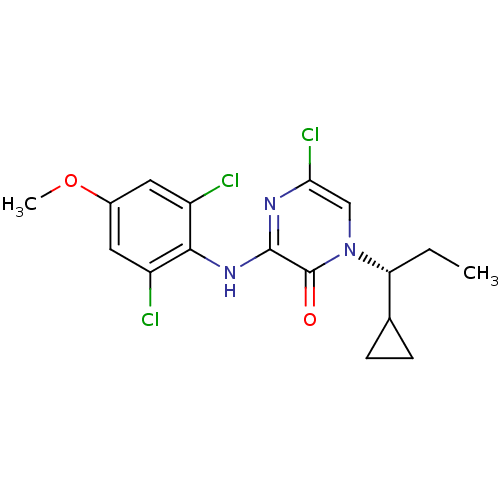 ((R)-5-Chloro-1-(1-cyclopropylpropyl)-3-(2,6-dichlo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]ovine-CRF from CRF1 receptor in rat frontal cortex by rapid filtration technique | J Med Chem 52: 4161-72 (2009) Article DOI: 10.1021/jm900302q BindingDB Entry DOI: 10.7270/Q2WW7JM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293935 ((R)-5-Chloro-1-(1-cyclopropylethyl)-3-[2,6-dichlor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]ovine-CRF from CRF1 receptor in rat frontal cortex by rapid filtration technique | J Med Chem 52: 4161-72 (2009) Article DOI: 10.1021/jm900302q BindingDB Entry DOI: 10.7270/Q2WW7JM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293973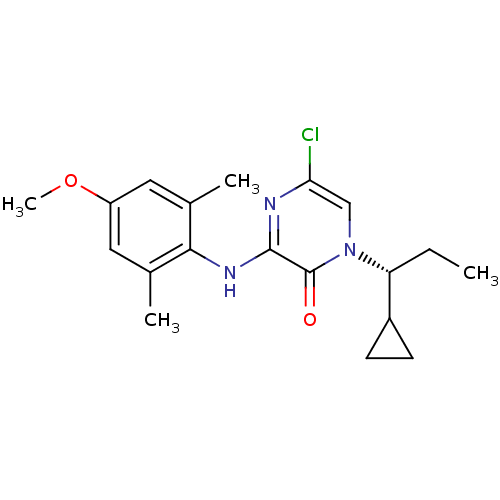 ((R)-5-Chloro-1-(1-cyclopropylpropyl)-3-(4-methoxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | J Med Chem 52: 4173-91 (2009) Article DOI: 10.1021/jm900301y BindingDB Entry DOI: 10.7270/Q2G44Q9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293914 ((R)-5-chloro-1-(1-cyclopropylpropyl)-3-(2,4-dichlo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | J Med Chem 52: 4173-91 (2009) Article DOI: 10.1021/jm900301y BindingDB Entry DOI: 10.7270/Q2G44Q9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293914 ((R)-5-chloro-1-(1-cyclopropylpropyl)-3-(2,4-dichlo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]ovine-CRF from CRF1 receptor in rat frontal cortex by rapid filtration technique | J Med Chem 52: 4161-72 (2009) Article DOI: 10.1021/jm900302q BindingDB Entry DOI: 10.7270/Q2WW7JM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin releasing hormone receptor 2 (Sus scrofa) | BDBM50158983 (CHEMBL439883 | E G P P I S I D L S L E L L R K M I...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]sauvagine from CRF2 receptor in pig choroid plexus by rapid filtration technique | J Med Chem 52: 4161-72 (2009) Article DOI: 10.1021/jm900302q BindingDB Entry DOI: 10.7270/Q2WW7JM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293953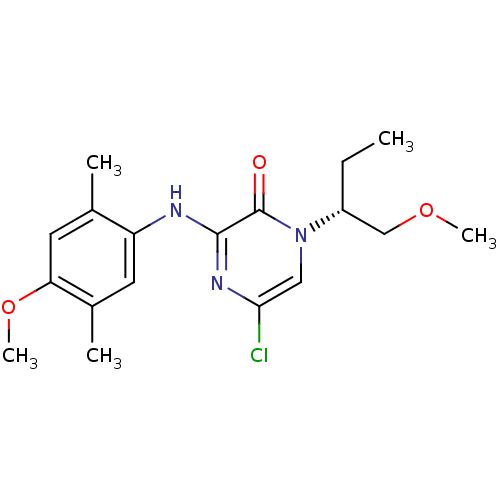 ((R)-5-Chloro-3-(4-methoxy-2,5-dimethylphenylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | J Med Chem 52: 4173-91 (2009) Article DOI: 10.1021/jm900301y BindingDB Entry DOI: 10.7270/Q2G44Q9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293952 (5-Chloro-3-(4-methoxy-2,5-dimethylphenylamino)-1-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | J Med Chem 52: 4173-91 (2009) Article DOI: 10.1021/jm900301y BindingDB Entry DOI: 10.7270/Q2G44Q9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293981 ((R)-5-Chloro-3-(7-chloro-5-methoxyindolin-1-yl)-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | J Med Chem 52: 4173-91 (2009) Article DOI: 10.1021/jm900301y BindingDB Entry DOI: 10.7270/Q2G44Q9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293913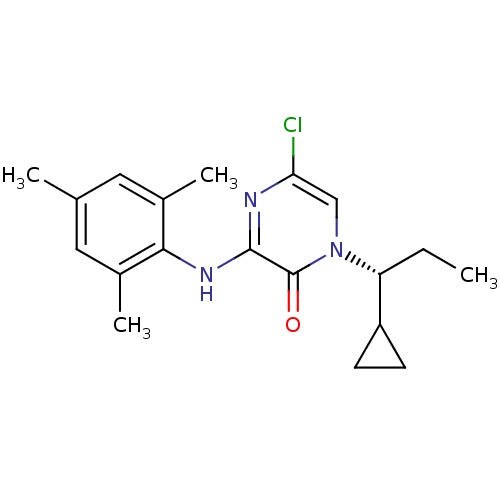 ((R)-5-Chloro-1-(1-cyclopropylpropyl)-3-(2,4,6-trim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | J Med Chem 52: 4173-91 (2009) Article DOI: 10.1021/jm900301y BindingDB Entry DOI: 10.7270/Q2G44Q9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293913 ((R)-5-Chloro-1-(1-cyclopropylpropyl)-3-(2,4,6-trim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]ovine-CRF from CRF1 receptor in rat frontal cortex by rapid filtration technique | J Med Chem 52: 4161-72 (2009) Article DOI: 10.1021/jm900302q BindingDB Entry DOI: 10.7270/Q2WW7JM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293951 (5-Chloro-1-(1-ethylpropyl)-3-(4-methoxy-2,5-dimeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | J Med Chem 52: 4173-91 (2009) Article DOI: 10.1021/jm900301y BindingDB Entry DOI: 10.7270/Q2G44Q9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293930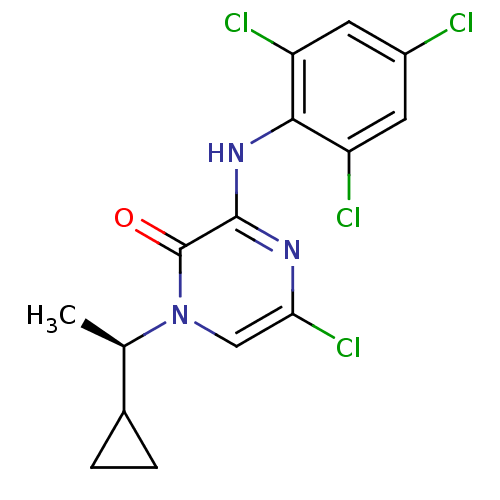 ((R)-5-Chloro-1-(1-cyclopropylethyl)-3-(2,4,6-trich...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]ovine-CRF from CRF1 receptor in rat frontal cortex by rapid filtration technique | J Med Chem 52: 4161-72 (2009) Article DOI: 10.1021/jm900302q BindingDB Entry DOI: 10.7270/Q2WW7JM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50529386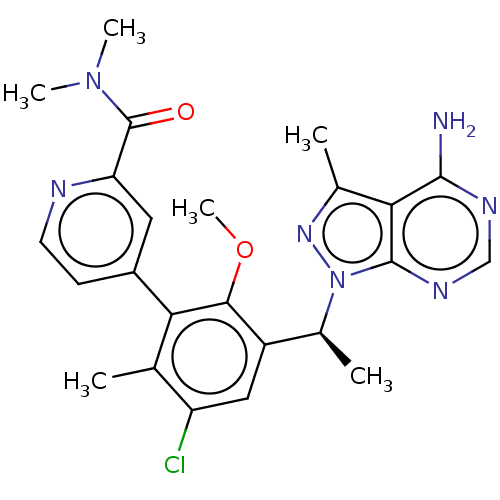 (CHEMBL4458634) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of PI3Kdelta (unknown origin) using D-myophosphatidylinositol 4,5-bisphosphate as substrate incubated for 120 mins by [gamma-33P]ATP based... | ACS Med Chem Lett 10: 1554-1560 (2019) Article DOI: 10.1021/acsmedchemlett.9b00334 BindingDB Entry DOI: 10.7270/Q2ST7T86 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293982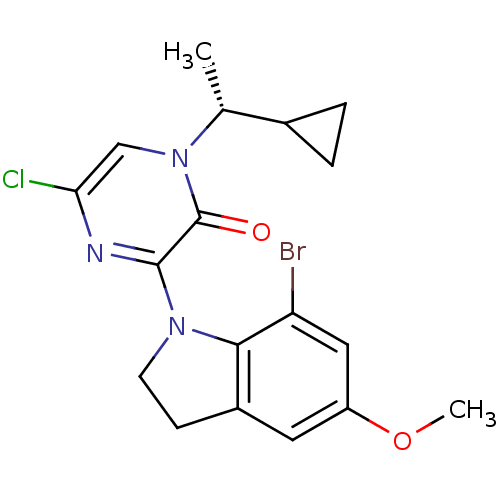 ((R)-3-(7-Bromo-5-methoxyindolin-1-yl)-5-chloro-1-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | J Med Chem 52: 4173-91 (2009) Article DOI: 10.1021/jm900301y BindingDB Entry DOI: 10.7270/Q2G44Q9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293929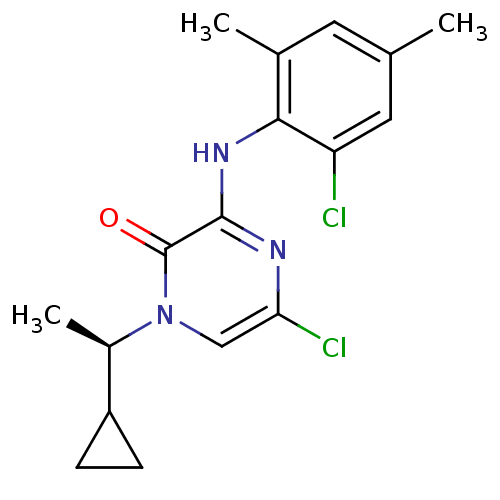 ((R)-5-Chloro-3-(2-chloro-4,6-dimethylphenylamino)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]ovine-CRF from CRF1 receptor in rat frontal cortex by rapid filtration technique | J Med Chem 52: 4161-72 (2009) Article DOI: 10.1021/jm900302q BindingDB Entry DOI: 10.7270/Q2WW7JM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50529384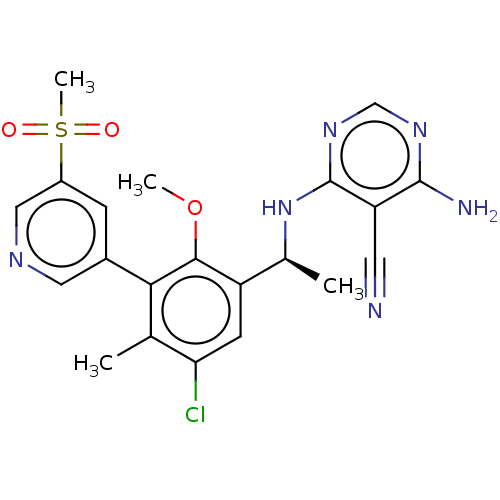 (CHEMBL4438376) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of PI3Kdelta (unknown origin) using D-myophosphatidylinositol 4,5-bisphosphate as substrate incubated for 120 mins by [gamma-33P]ATP based... | ACS Med Chem Lett 10: 1554-1560 (2019) Article DOI: 10.1021/acsmedchemlett.9b00334 BindingDB Entry DOI: 10.7270/Q2ST7T86 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM272573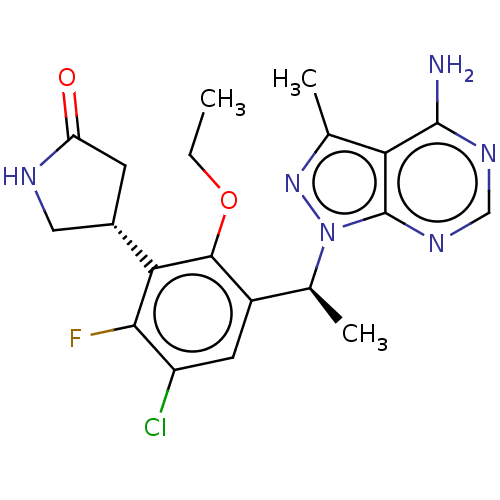 (US10065963, 32c | US10376513, Example 346 | US1064...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of PI3Kdelta (unknown origin) using D-myophosphatidylinositol 4,5-bisphosphate as substrate incubated for 120 mins by [gamma-33P]ATP based... | ACS Med Chem Lett 10: 1554-1560 (2019) Article DOI: 10.1021/acsmedchemlett.9b00334 BindingDB Entry DOI: 10.7270/Q2ST7T86 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM261330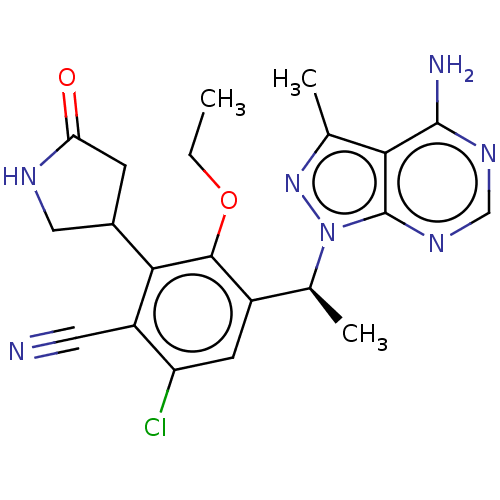 (US10092570, Example 352 | US9707233, 350) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of PI3Kdelta (unknown origin) using D-myophosphatidylinositol 4,5-bisphosphate as substrate incubated for 120 mins by [gamma-33P]ATP based... | ACS Med Chem Lett 10: 1554-1560 (2019) Article DOI: 10.1021/acsmedchemlett.9b00334 BindingDB Entry DOI: 10.7270/Q2ST7T86 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293910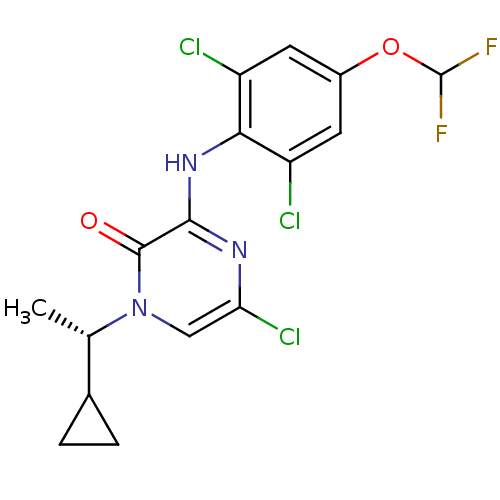 ((S)-5-chloro-1-(1-cyclopropylethyl)-3-(2,6-dichlor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]ovine-CRF from CRF1 receptor in rat frontal cortex by rapid filtration technique | J Med Chem 52: 4161-72 (2009) Article DOI: 10.1021/jm900302q BindingDB Entry DOI: 10.7270/Q2WW7JM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM272573 (US10065963, 32c | US10376513, Example 346 | US1064...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of PI3Kdelta (unknown origin) using D-myophosphatidylinositol 4,5-bisphosphate as substrate incubated for 120 mins by [gamma-33P]-ATP base... | ACS Med Chem Lett 10: 1554-1560 (2019) Article DOI: 10.1021/acsmedchemlett.9b00334 BindingDB Entry DOI: 10.7270/Q2ST7T86 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293927 ((R)-5-Chloro-1-(1-cyclopropylethyl)-3-(2,6-dichlor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]ovine-CRF from CRF1 receptor in rat frontal cortex by rapid filtration technique | J Med Chem 52: 4161-72 (2009) Article DOI: 10.1021/jm900302q BindingDB Entry DOI: 10.7270/Q2WW7JM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293927 ((R)-5-Chloro-1-(1-cyclopropylethyl)-3-(2,6-dichlor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | J Med Chem 52: 4173-91 (2009) Article DOI: 10.1021/jm900301y BindingDB Entry DOI: 10.7270/Q2G44Q9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM272573 (US10065963, 32c | US10376513, Example 346 | US1064...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of PI3Kdelta in human Ramos cells assessed as reduction in AKT phosphorylation incubated for 2 hrs by Alexa flour 488 based FACS analysis | ACS Med Chem Lett 10: 1554-1560 (2019) Article DOI: 10.1021/acsmedchemlett.9b00334 BindingDB Entry DOI: 10.7270/Q2ST7T86 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293915 ((R)-3,5-Dichloro-4-[6-chloro-4-(1-cyclopropylpropy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | J Med Chem 52: 4173-91 (2009) Article DOI: 10.1021/jm900301y BindingDB Entry DOI: 10.7270/Q2G44Q9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293915 ((R)-3,5-Dichloro-4-[6-chloro-4-(1-cyclopropylpropy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]ovine-CRF from CRF1 receptor in rat frontal cortex by rapid filtration technique | J Med Chem 52: 4161-72 (2009) Article DOI: 10.1021/jm900302q BindingDB Entry DOI: 10.7270/Q2WW7JM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293986 ((R)-5-Bromo-1-(1-cyclopropylethyl)-3-[6-methoxy-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | J Med Chem 52: 4173-91 (2009) Article DOI: 10.1021/jm900301y BindingDB Entry DOI: 10.7270/Q2G44Q9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50084875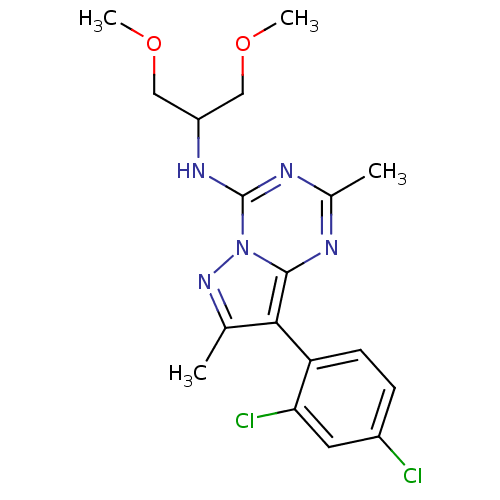 (8-(2,4-dichlorophenyl)-N-(1,3-dimethoxypropan-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]ovine-CRF from CRF1 receptor in rat frontal cortex by rapid filtration technique | J Med Chem 52: 4161-72 (2009) Article DOI: 10.1021/jm900302q BindingDB Entry DOI: 10.7270/Q2WW7JM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293937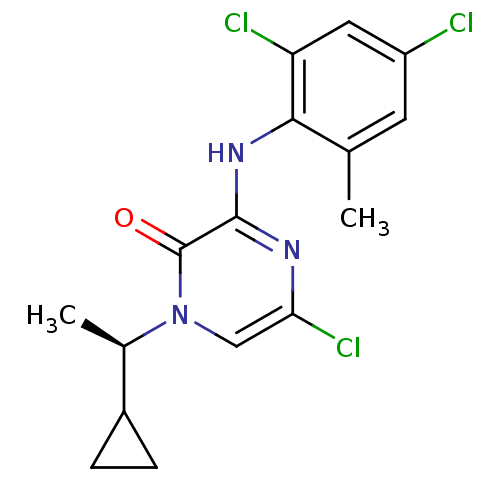 ((R)-5-Chloro-1-(1-cyclopropylethyl)-3-(2,4-dichlor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]ovine-CRF from CRF1 receptor in rat frontal cortex by rapid filtration technique | J Med Chem 52: 4161-72 (2009) Article DOI: 10.1021/jm900302q BindingDB Entry DOI: 10.7270/Q2WW7JM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293942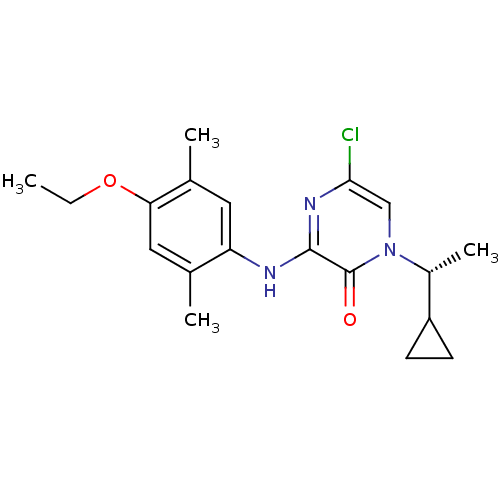 ((R)-5-Chloro-1-(1-cyclopropylethyl)-3-(4-ethoxy-2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]ovine-CRF from CRF1 receptor in rat frontal cortex by rapid filtration technique | J Med Chem 52: 4161-72 (2009) Article DOI: 10.1021/jm900302q BindingDB Entry DOI: 10.7270/Q2WW7JM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293922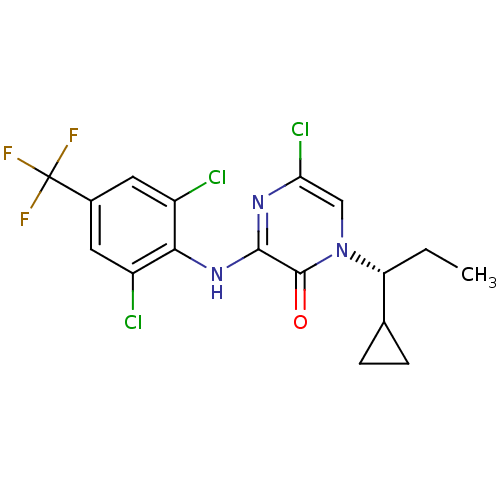 ((R)-5-Chloro-1-(1-cyclopropylpropyl)-3-(2,6-dichlo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]ovine-CRF from CRF1 receptor in rat frontal cortex by rapid filtration technique | J Med Chem 52: 4161-72 (2009) Article DOI: 10.1021/jm900302q BindingDB Entry DOI: 10.7270/Q2WW7JM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293959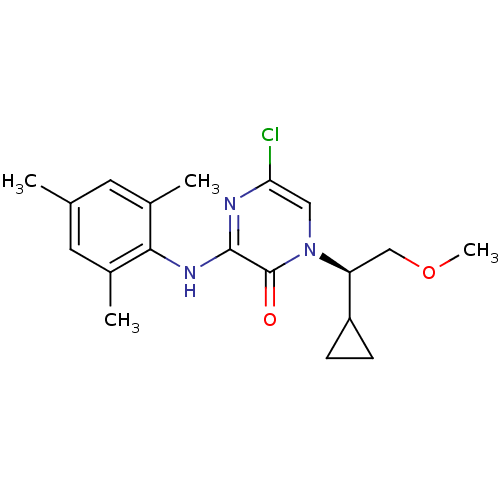 ((R)-5-Chloro-1-(1-cyclopropyl-2-methoxyethyl)-3-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | J Med Chem 52: 4173-91 (2009) Article DOI: 10.1021/jm900301y BindingDB Entry DOI: 10.7270/Q2G44Q9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM261317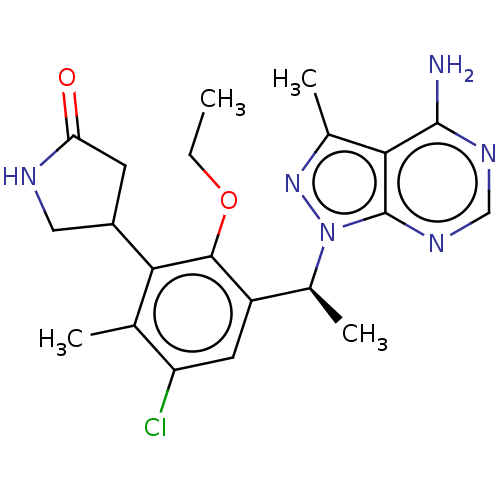 (US10376513, Example 310 | US11433071, Example 311 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of PI3Kdelta (unknown origin) using D-myophosphatidylinositol 4,5-bisphosphate as substrate incubated for 120 mins by [gamma-33P]ATP based... | ACS Med Chem Lett 10: 1554-1560 (2019) Article DOI: 10.1021/acsmedchemlett.9b00334 BindingDB Entry DOI: 10.7270/Q2ST7T86 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293960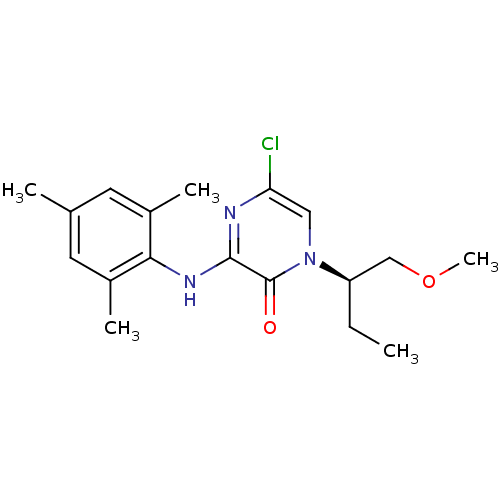 ((R)-5-Chloro-1-[1-(methoxymethyl)propyl]-3-(2,4,6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | J Med Chem 52: 4173-91 (2009) Article DOI: 10.1021/jm900301y BindingDB Entry DOI: 10.7270/Q2G44Q9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293975 ((R)-5-Chloro-1-(1-cyclopropyl-2-methoxyethyl)-3-(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | J Med Chem 52: 4173-91 (2009) Article DOI: 10.1021/jm900301y BindingDB Entry DOI: 10.7270/Q2G44Q9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293978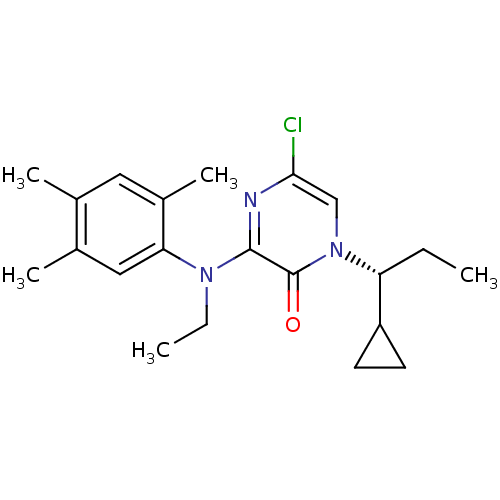 ((R)-5-Chloro-1-(1-cyclopropylpropyl)-3-[ethyl(2,4,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate by gamma counting | J Med Chem 52: 4173-91 (2009) Article DOI: 10.1021/jm900301y BindingDB Entry DOI: 10.7270/Q2G44Q9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293925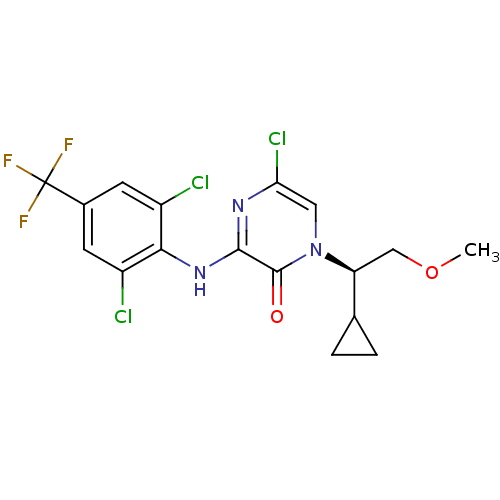 ((R)-5-Chloro-1-(1-cyclopropyl-2-methoxyethyl)-3-[2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]ovine-CRF from CRF1 receptor in rat frontal cortex by rapid filtration technique | J Med Chem 52: 4161-72 (2009) Article DOI: 10.1021/jm900302q BindingDB Entry DOI: 10.7270/Q2WW7JM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13469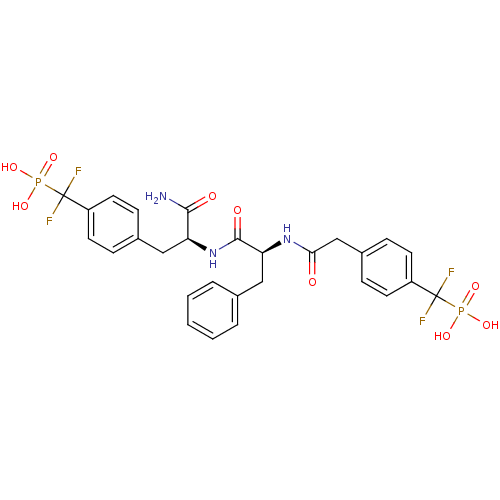 (({4-[(2S)-2-carbamoyl-2-[(2S)-2-(1-{4-[difluoro(ph...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 38013-21 (2006) Article DOI: 10.1074/jbc.M607913200 BindingDB Entry DOI: 10.7270/Q2JW8C4X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50529381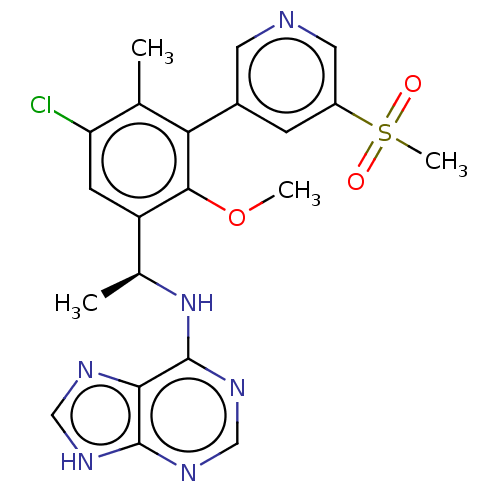 (CHEMBL4446120) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of PI3Kdelta (unknown origin) using D-myophosphatidylinositol 4,5-bisphosphate as substrate incubated for 120 mins by [gamma-33P]ATP based... | ACS Med Chem Lett 10: 1554-1560 (2019) Article DOI: 10.1021/acsmedchemlett.9b00334 BindingDB Entry DOI: 10.7270/Q2ST7T86 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50293920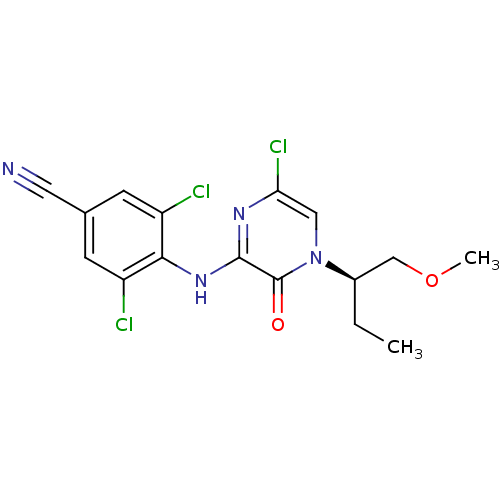 ((R)-3,5-Dichloro-4-{6-chloro-4-[1-(methoxymethyl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]ovine-CRF from CRF1 receptor in rat frontal cortex by rapid filtration technique | J Med Chem 52: 4161-72 (2009) Article DOI: 10.1021/jm900302q BindingDB Entry DOI: 10.7270/Q2WW7JM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 5316 total ) | Next | Last >> |