

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM85530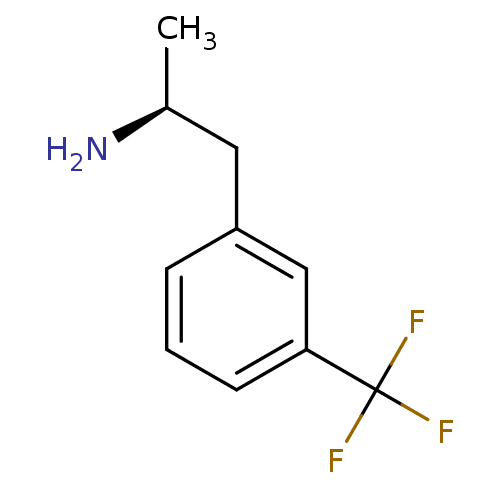 (Nor-d-fenfluramine | Nor-dexfenfluramine | Norfenf...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Research Laboratories Curated by PDSP Ki Database | Mol Pharmacol 57: 75-81 (2000) BindingDB Entry DOI: 10.7270/Q2P26WPH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM85530 (Nor-d-fenfluramine | Nor-dexfenfluramine | Norfenf...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Research Laboratories Curated by PDSP Ki Database | Mol Pharmacol 57: 75-81 (2000) BindingDB Entry DOI: 10.7270/Q2P26WPH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM85598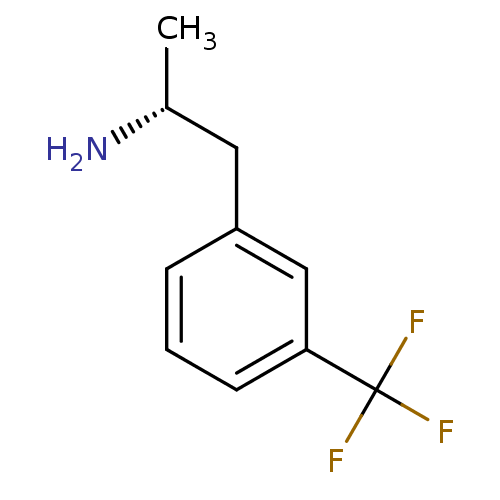 (l-norfenfluramine) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Research Laboratories Curated by PDSP Ki Database | Mol Pharmacol 57: 75-81 (2000) BindingDB Entry DOI: 10.7270/Q2P26WPH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM85598 (l-norfenfluramine) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Research Laboratories Curated by PDSP Ki Database | Mol Pharmacol 57: 75-81 (2000) BindingDB Entry DOI: 10.7270/Q2P26WPH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM85530 (Nor-d-fenfluramine | Nor-dexfenfluramine | Norfenf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | PubMed | 187 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Research Laboratories Curated by PDSP Ki Database | Mol Pharmacol 57: 75-81 (2000) BindingDB Entry DOI: 10.7270/Q2P26WPH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM85598 (l-norfenfluramine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 267 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Research Laboratories Curated by PDSP Ki Database | Mol Pharmacol 57: 75-81 (2000) BindingDB Entry DOI: 10.7270/Q2P26WPH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM85597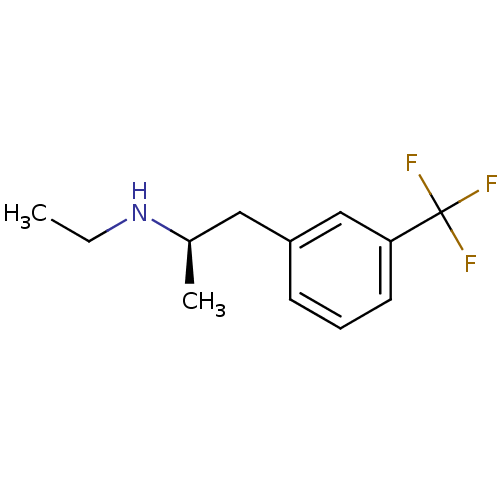 (CAS_37577-24-5 | NSC_65801 | l-Fenfluramine) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents Similars | PubMed | 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Research Laboratories Curated by PDSP Ki Database | Mol Pharmacol 57: 75-81 (2000) BindingDB Entry DOI: 10.7270/Q2P26WPH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM85597 (CAS_37577-24-5 | NSC_65801 | l-Fenfluramine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents Similars | PubMed | 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Research Laboratories Curated by PDSP Ki Database | Mol Pharmacol 57: 75-81 (2000) BindingDB Entry DOI: 10.7270/Q2P26WPH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM85597 (CAS_37577-24-5 | NSC_65801 | l-Fenfluramine) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents Similars | PubMed | 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Research Laboratories Curated by PDSP Ki Database | Mol Pharmacol 57: 75-81 (2000) BindingDB Entry DOI: 10.7270/Q2P26WPH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM85596 (CAS_3239-45-0 | NSC_65801 | d-Fenfluramine) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Patents Similars | DrugBank PubMed | 2.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Research Laboratories Curated by PDSP Ki Database | Mol Pharmacol 57: 75-81 (2000) BindingDB Entry DOI: 10.7270/Q2P26WPH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM85596 (CAS_3239-45-0 | NSC_65801 | d-Fenfluramine) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Patents Similars | PubMed | 3.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Research Laboratories Curated by PDSP Ki Database | Mol Pharmacol 57: 75-81 (2000) BindingDB Entry DOI: 10.7270/Q2P26WPH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13467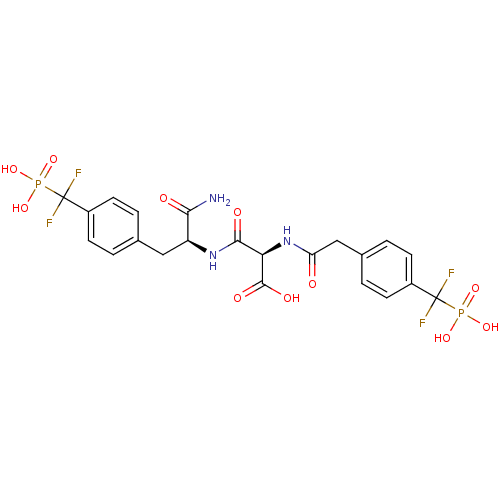 ((2R)-2-{[(1S)-1-carbamoyl-2-{4-[difluoro(phosphono...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 38013-21 (2006) Article DOI: 10.1074/jbc.M607913200 BindingDB Entry DOI: 10.7270/Q2JW8C4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13469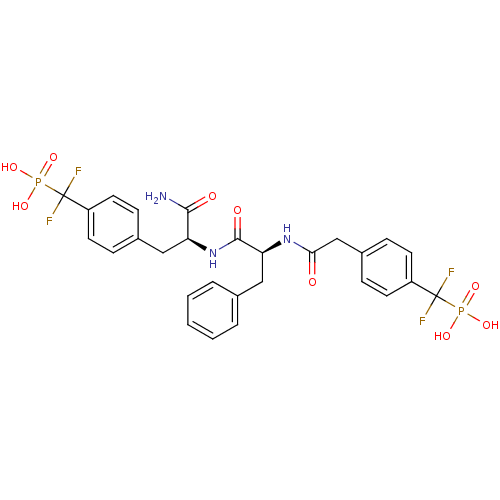 (({4-[(2S)-2-carbamoyl-2-[(2S)-2-(1-{4-[difluoro(ph...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 38013-21 (2006) Article DOI: 10.1074/jbc.M607913200 BindingDB Entry DOI: 10.7270/Q2JW8C4X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM246870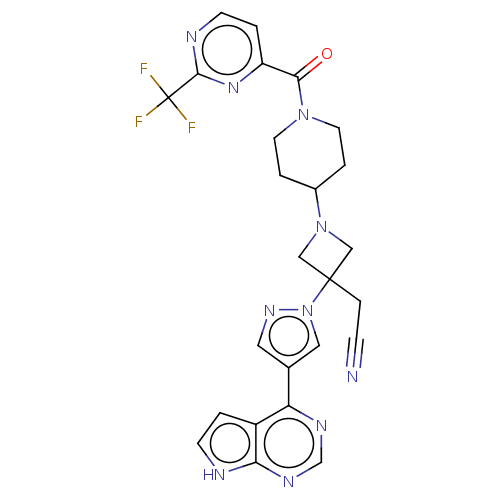 (US10053465, 9 | US10065963, Compound 9 | US1012515...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description JAK1 pathway inhibitors that can be used for the treatment of cytokine-related diseases or disorders are tested for inhibitory activity of JAK target... | Citation and Details BindingDB Entry DOI: 10.7270/Q27M0CVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM246886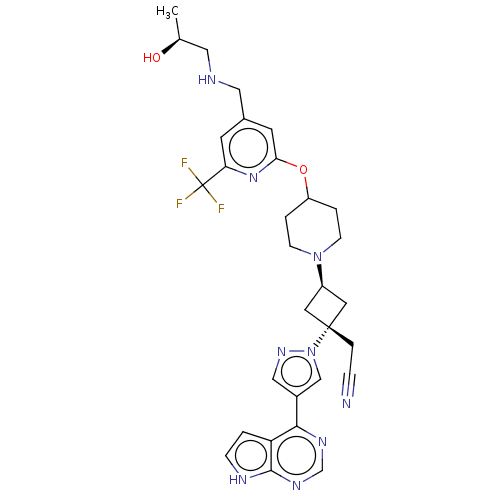 (US10053465, 25 | US10077277, Example 25 | US101251...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description JAK1 pathway inhibitors that can be used for the treatment of cytokine-related diseases or disorders are tested for inhibitory activity of JAK target... | Citation and Details BindingDB Entry DOI: 10.7270/Q27M0CVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM246885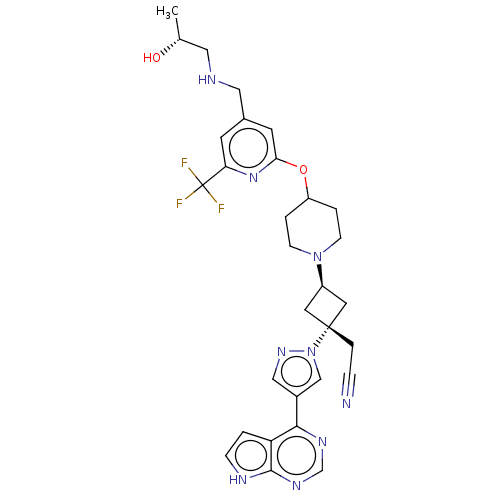 (US10053465, 24 | US10065963, Compound 25 | US10125...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description JAK1 pathway inhibitors that can be used for the treatment of cytokine-related diseases or disorders are tested for inhibitory activity of JAK target... | Citation and Details BindingDB Entry DOI: 10.7270/Q27M0CVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM246884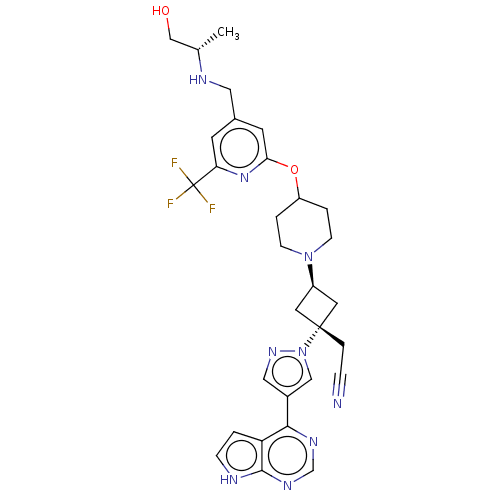 (US10053465, 23 | US10065963, Compound 23 | US10125...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description JAK1 pathway inhibitors that can be used for the treatment of cytokine-related diseases or disorders are tested for inhibitory activity of JAK target... | Citation and Details BindingDB Entry DOI: 10.7270/Q27M0CVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM246883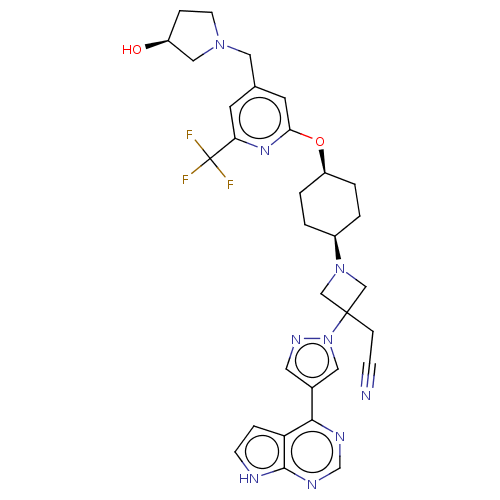 (US10005788, 1 (2nd peak, cis-) | US10053465, 22 | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description JAK1 pathway inhibitors that can be used for the treatment of cytokine-related diseases or disorders are tested for inhibitory activity of JAK target... | Citation and Details BindingDB Entry DOI: 10.7270/Q27M0CVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM246882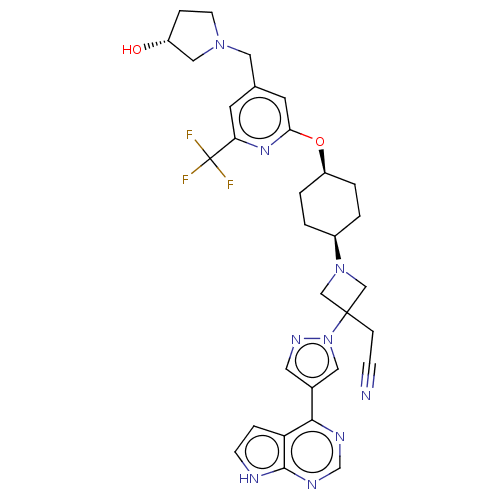 (US10053465, 21 | US10065963, Compound 21 | US10125...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description JAK1 pathway inhibitors that can be used for the treatment of cytokine-related diseases or disorders are tested for inhibitory activity of JAK target... | Citation and Details BindingDB Entry DOI: 10.7270/Q27M0CVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM246881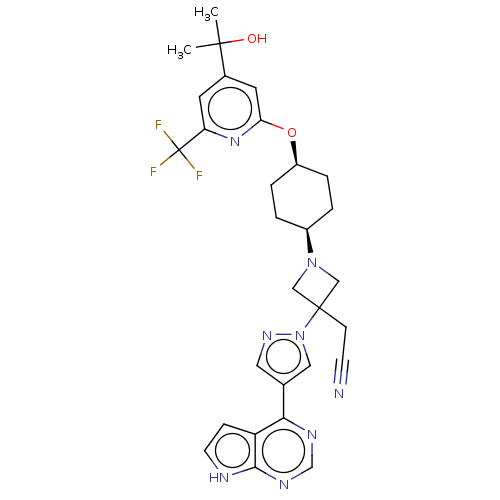 (US10053465, 20 | US10065963, Compound 20 | US10125...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description JAK1 pathway inhibitors that can be used for the treatment of cytokine-related diseases or disorders are tested for inhibitory activity of JAK target... | Citation and Details BindingDB Entry DOI: 10.7270/Q27M0CVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM246880 (US10053465, 19 | US10065963, Compound 19 | US10125...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description JAK1 pathway inhibitors that can be used for the treatment of cytokine-related diseases or disorders are tested for inhibitory activity of JAK target... | Citation and Details BindingDB Entry DOI: 10.7270/Q27M0CVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM246879 (US10053465, 18 | US10065963, Compound 18 | US10125...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description JAK1 pathway inhibitors that can be used for the treatment of cytokine-related diseases or disorders are tested for inhibitory activity of JAK target... | Citation and Details BindingDB Entry DOI: 10.7270/Q27M0CVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM262166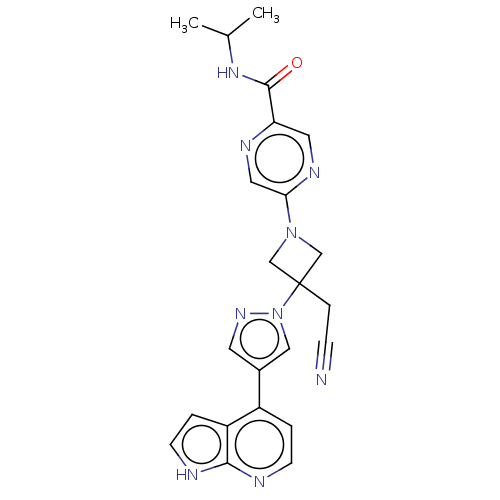 (5-{3-(cyanomethyl)- 3-[4-(1H-pyrrolo[2,3- b]pyridi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description JAK1 pathway inhibitors that can be used for the treatment of cytokine-related diseases or disorders are tested for inhibitory activity of JAK target... | Citation and Details BindingDB Entry DOI: 10.7270/Q27M0CVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM596393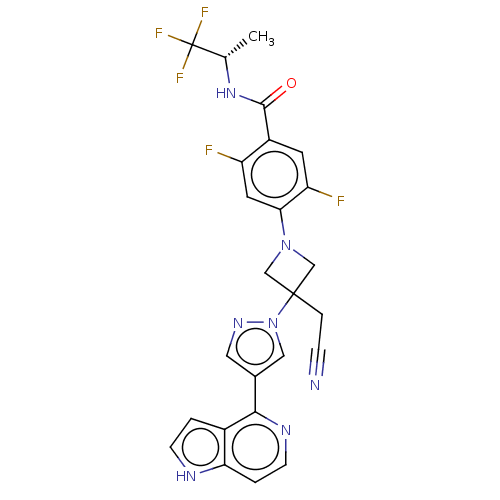 (4-{3-(cyanomethyl)-3-[4- (7H-pyrrolo[2,3- d]pyrimi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description JAK1 pathway inhibitors that can be used for the treatment of cytokine-related diseases or disorders are tested for inhibitory activity of JAK target... | Citation and Details BindingDB Entry DOI: 10.7270/Q27M0CVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM596392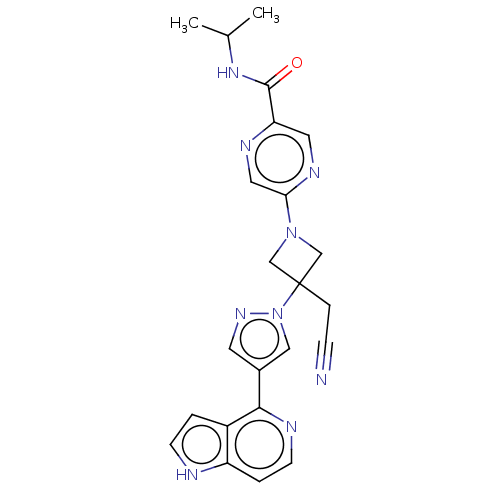 (5-{3-(cyanomethyl)-3-[4- (7H-pyrrolo[2,3- d]pyrimi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description JAK1 pathway inhibitors that can be used for the treatment of cytokine-related diseases or disorders are tested for inhibitory activity of JAK target... | Citation and Details BindingDB Entry DOI: 10.7270/Q27M0CVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM246875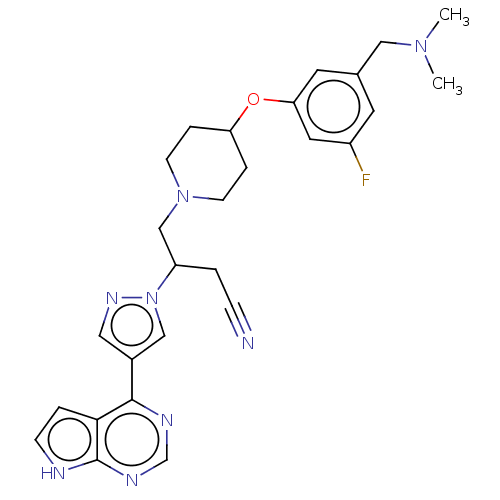 (4-(4-{3- [(dimethylamino) methyl]-5- fluorophenoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description JAK1 pathway inhibitors that can be used for the treatment of cytokine-related diseases or disorders are tested for inhibitory activity of JAK target... | Citation and Details BindingDB Entry DOI: 10.7270/Q27M0CVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM246874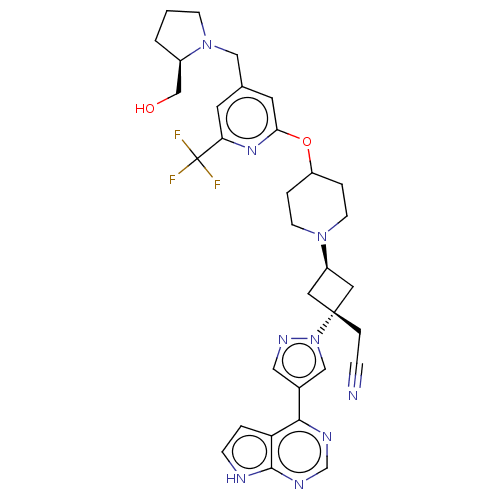 (US10053465, 13 | US10065963, Compound 13 | US10125...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description JAK1 pathway inhibitors that can be used for the treatment of cytokine-related diseases or disorders are tested for inhibitory activity of JAK target... | Citation and Details BindingDB Entry DOI: 10.7270/Q27M0CVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM246873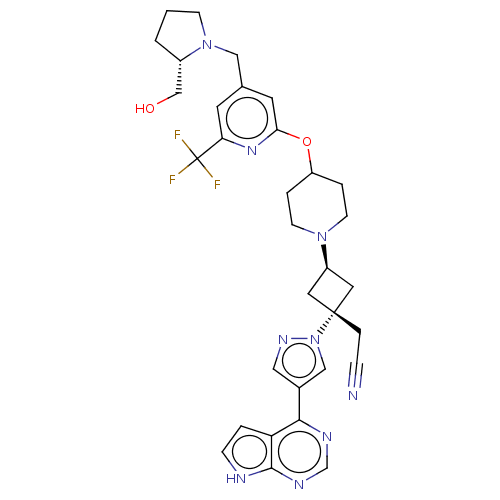 (US10053465, 12 | US10065963, Compound 12 | US10125...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description JAK1 pathway inhibitors that can be used for the treatment of cytokine-related diseases or disorders are tested for inhibitory activity of JAK target... | Citation and Details BindingDB Entry DOI: 10.7270/Q27M0CVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM246872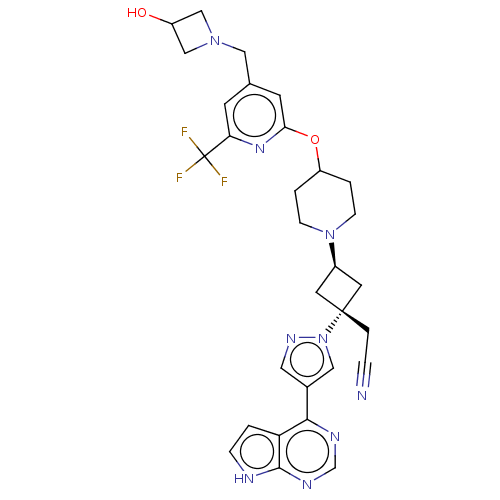 (US10053465, 11 | US10065963, Compound 11 | US10125...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description JAK1 pathway inhibitors that can be used for the treatment of cytokine-related diseases or disorders are tested for inhibitory activity of JAK target... | Citation and Details BindingDB Entry DOI: 10.7270/Q27M0CVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM246871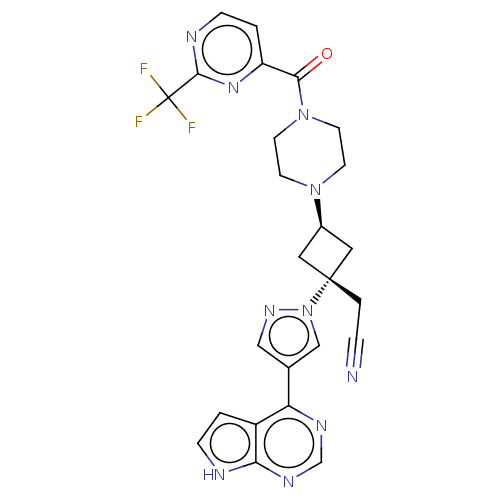 (US10053465, 10 | US10065963, Compound 10 | US10125...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description JAK1 pathway inhibitors that can be used for the treatment of cytokine-related diseases or disorders are tested for inhibitory activity of JAK target... | Citation and Details BindingDB Entry DOI: 10.7270/Q27M0CVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM246867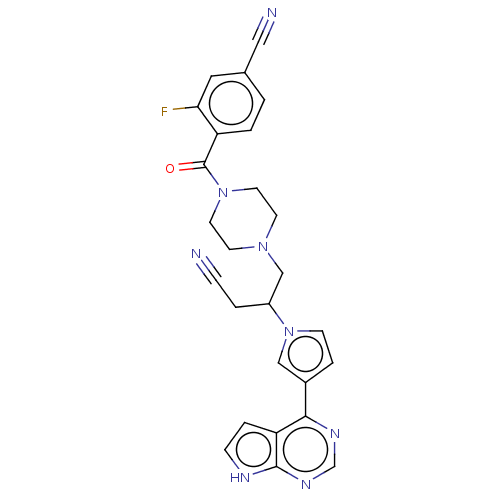 (4-[(4-{3-cyano-2-[3- (7H-pyrrolo[2,3- d]pyrimidin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description JAK1 pathway inhibitors that can be used for the treatment of cytokine-related diseases or disorders are tested for inhibitory activity of JAK target... | Citation and Details BindingDB Entry DOI: 10.7270/Q27M0CVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM596385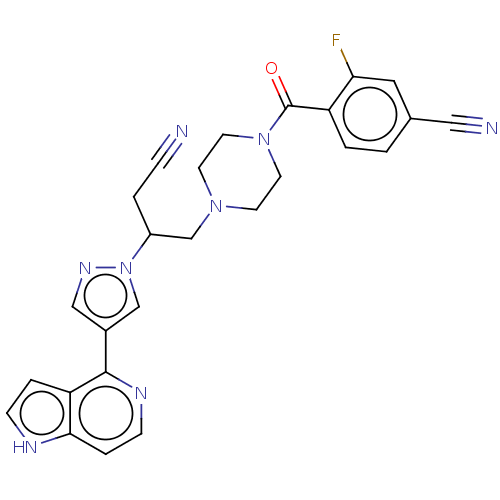 (4-[(4-{3-cyano-2-[4-(7H- pyrrolo[2,3-d]pyrimidin- ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description JAK1 pathway inhibitors that can be used for the treatment of cytokine-related diseases or disorders are tested for inhibitory activity of JAK target... | Citation and Details BindingDB Entry DOI: 10.7270/Q27M0CVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM246864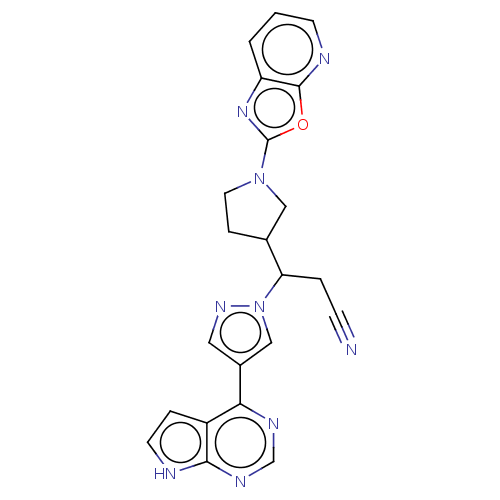 (3-(1- [1,3]oxazolo[5,4- b]pyridin-2- ylpyrrolidin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description JAK1 pathway inhibitors that can be used for the treatment of cytokine-related diseases or disorders are tested for inhibitory activity of JAK target... | Citation and Details BindingDB Entry DOI: 10.7270/Q27M0CVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM246861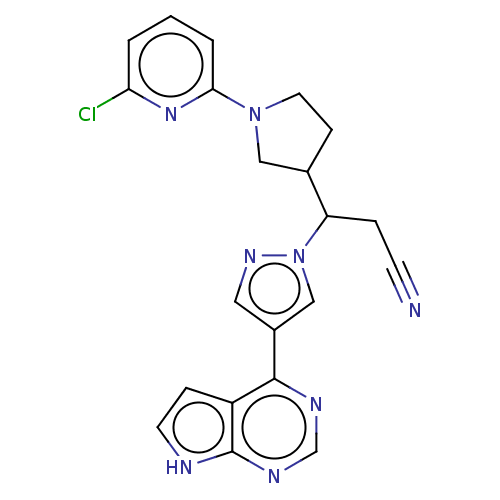 (3-[1-(6- chloropyridin-2- yl)pyrrolidin-3-yl]-3- [...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description JAK1 pathway inhibitors that can be used for the treatment of cytokine-related diseases or disorders are tested for inhibitory activity of JAK target... | Citation and Details BindingDB Entry DOI: 10.7270/Q27M0CVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM246888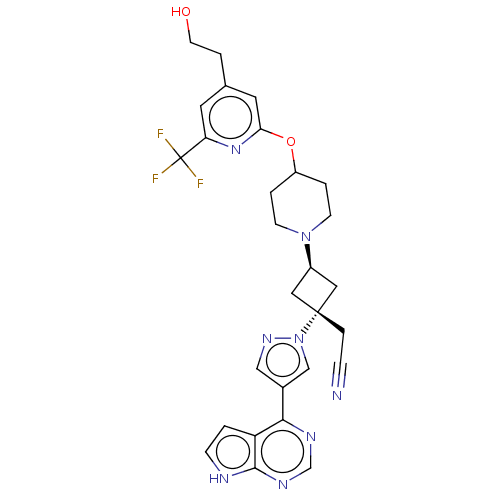 (US10053465, 26 | US10065963, Compound 26 | US10125...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description JAK1 pathway inhibitors that can be used for the treatment of cytokine-related diseases or disorders are tested for inhibitory activity of JAK target... | Citation and Details BindingDB Entry DOI: 10.7270/Q27M0CVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM246868 (US10053465, 7 | US10065963, Compound 7 | US1012515...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description JAK1 pathway inhibitors that can be used for the treatment of cytokine-related diseases or disorders are tested for inhibitory activity of JAK target... | Citation and Details BindingDB Entry DOI: 10.7270/Q27M0CVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM246869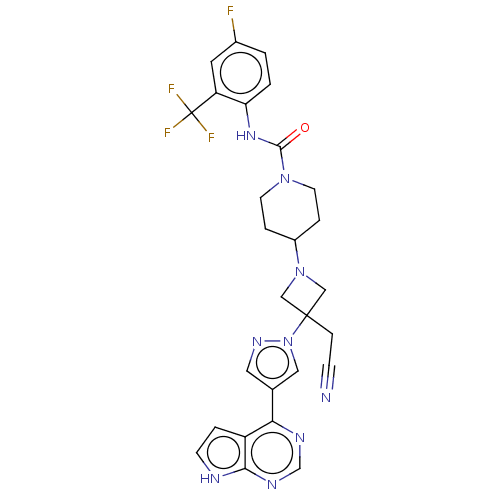 (4-{3-(Cyanomethyl)- 3-[4-(7H-pyrrolo[2,3- d]pyrimi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description JAK1 pathway inhibitors that can be used for the treatment of cytokine-related diseases or disorders are tested for inhibitory activity of JAK target... | Citation and Details BindingDB Entry DOI: 10.7270/Q27M0CVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13507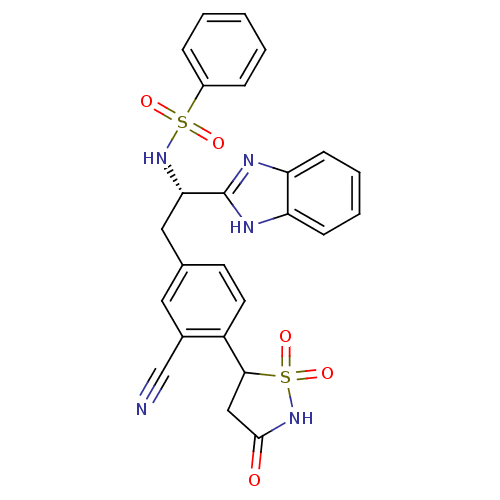 (Isothiazolidinone (IZD) deriv. 43 | N-[(1S)-1-(1H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 38013-21 (2006) Article DOI: 10.1074/jbc.M607913200 BindingDB Entry DOI: 10.7270/Q2JW8C4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13508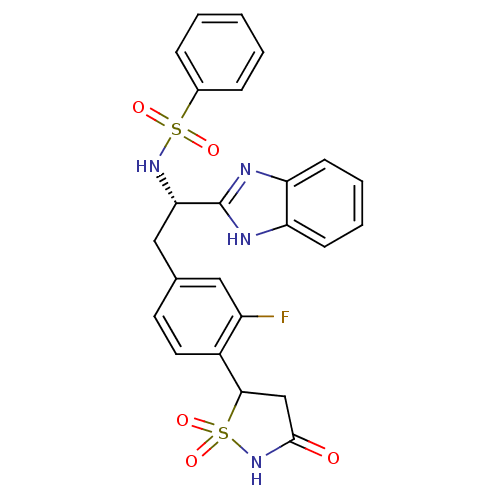 (Isothiazolidinone (IZD) deriv. 44 | N-[(1S)-1-(1H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 38013-21 (2006) Article DOI: 10.1074/jbc.M607913200 BindingDB Entry DOI: 10.7270/Q2JW8C4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13493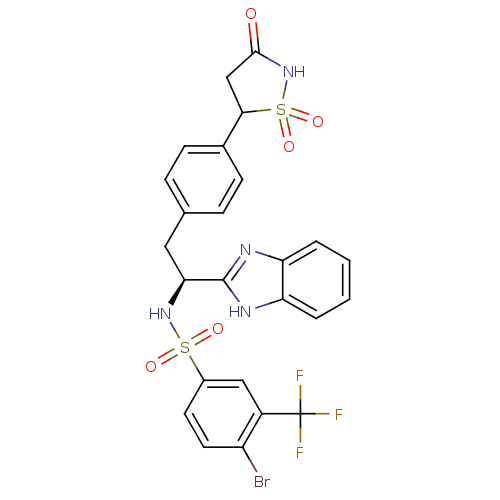 (Isothiazolidinone (IZD) deriv. 29 | N-[(1S)-1-(1H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 38013-21 (2006) Article DOI: 10.1074/jbc.M607913200 BindingDB Entry DOI: 10.7270/Q2JW8C4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13471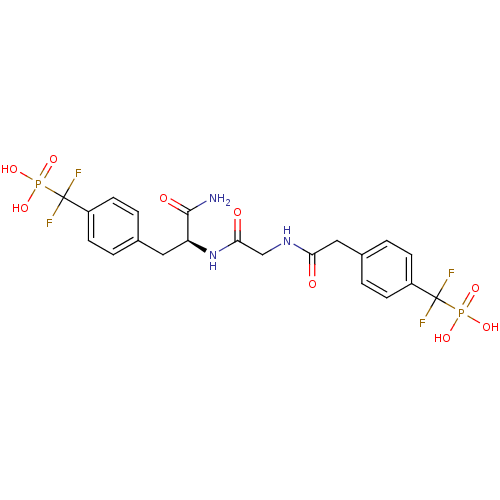 (({4-[(2S)-2-carbamoyl-2-[2-(1-{4-[difluoro(phospho...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 38013-21 (2006) Article DOI: 10.1074/jbc.M607913200 BindingDB Entry DOI: 10.7270/Q2JW8C4X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13470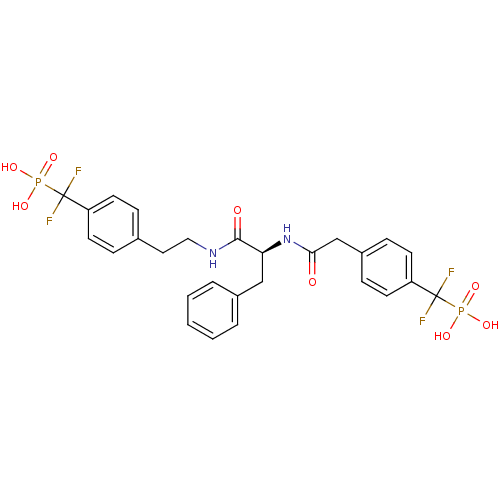 (Difluoromethylphosphonic acid (DFMP) deriv. 9 | [(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 38013-21 (2006) Article DOI: 10.1074/jbc.M607913200 BindingDB Entry DOI: 10.7270/Q2JW8C4X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13494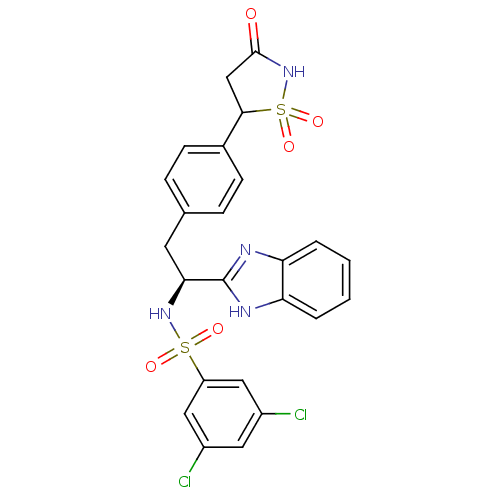 (Isothiazolidinone (IZD) deriv. 30 | N-[(1S)-1-(1H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 38013-21 (2006) Article DOI: 10.1074/jbc.M607913200 BindingDB Entry DOI: 10.7270/Q2JW8C4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13509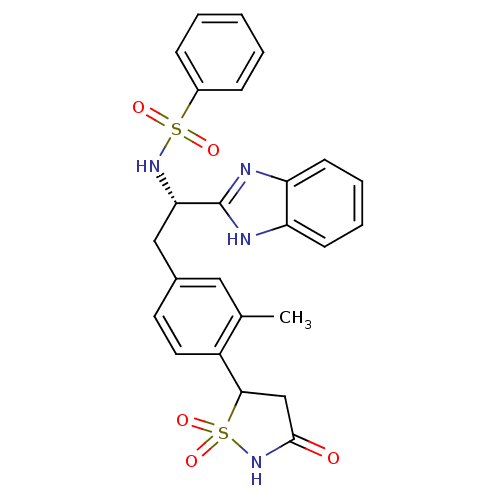 (Isothiazolidinone (IZD) deriv. 45 | N-[(1S)-1-(1H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 38013-21 (2006) Article DOI: 10.1074/jbc.M607913200 BindingDB Entry DOI: 10.7270/Q2JW8C4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13475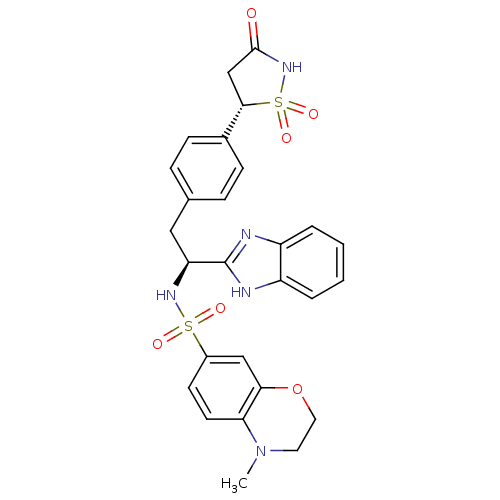 (Isothiazolidinone (IZD) deriv. 4 | N-[(1S)-1-(1H-1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 38013-21 (2006) Article DOI: 10.1074/jbc.M607913200 BindingDB Entry DOI: 10.7270/Q2JW8C4X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13809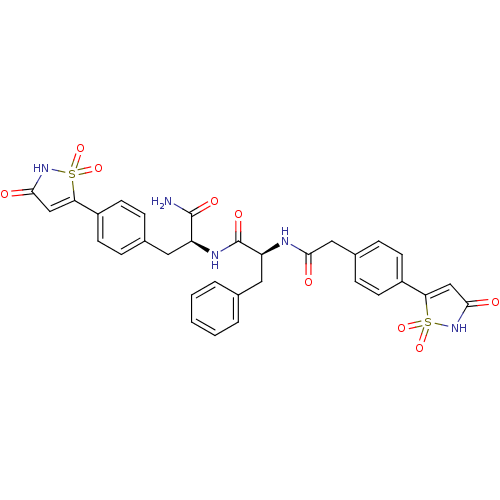 ((2S)-N-[(1S)-1-carbamoyl-2-[4-(1,1,3-trioxo-2,3-di...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 32784-95 (2006) Article DOI: 10.1074/jbc.M606873200 BindingDB Entry DOI: 10.7270/Q2GX48SD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13495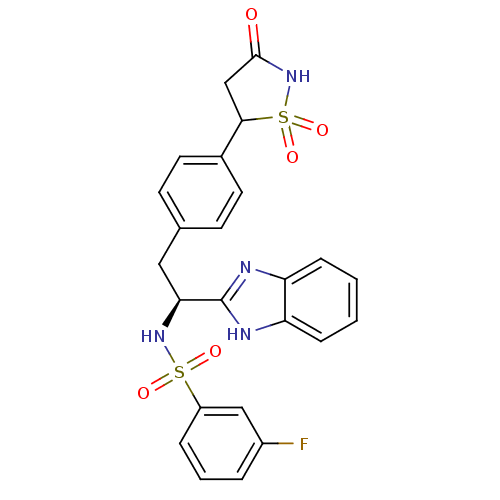 (Isothiazolidinone (IZD) deriv. 31 | N-[(1S)-1-(1H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 38013-21 (2006) Article DOI: 10.1074/jbc.M607913200 BindingDB Entry DOI: 10.7270/Q2JW8C4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13510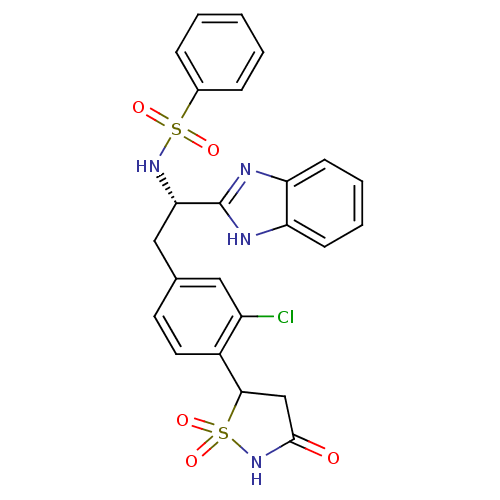 (Isothiazolidinone (IZD) deriv. 46 | N-[(1S)-1-(1H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 38013-21 (2006) Article DOI: 10.1074/jbc.M607913200 BindingDB Entry DOI: 10.7270/Q2JW8C4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13496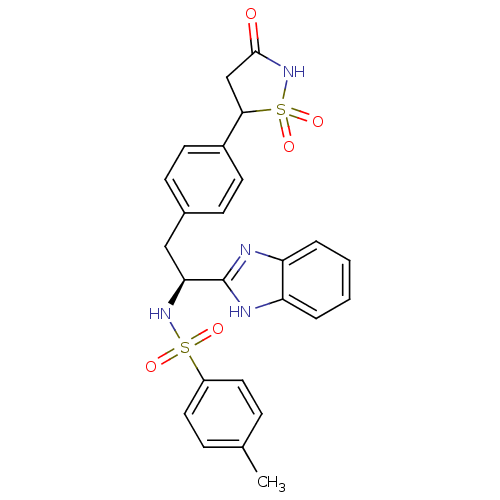 (Isothiazolidinone (IZD) deriv. 32 | N-[(1S)-1-(1H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 38013-21 (2006) Article DOI: 10.1074/jbc.M607913200 BindingDB Entry DOI: 10.7270/Q2JW8C4X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13497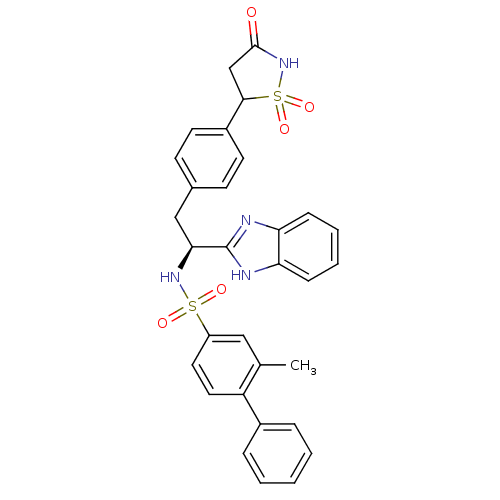 (Isothiazolidinone (IZD) deriv. 33 | N-[(1S)-1-(1H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 38013-21 (2006) Article DOI: 10.1074/jbc.M607913200 BindingDB Entry DOI: 10.7270/Q2JW8C4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 92 total ) | Next | Last >> |