 Found 595 hits with Last Name = 'burn' and Initial = 'tc'
Found 595 hits with Last Name = 'burn' and Initial = 'tc' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50065807
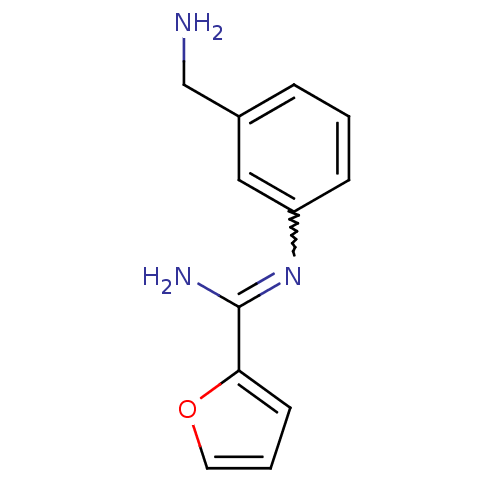
(CHEMBL552825 | N-(3-Aminomethyl-phenyl)-furan-2-ca...)Show InChI InChI=1S/C12H13N3O/c13-8-9-3-1-4-10(7-9)15-12(14)11-5-2-6-16-11/h1-7H,8,13H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50065843
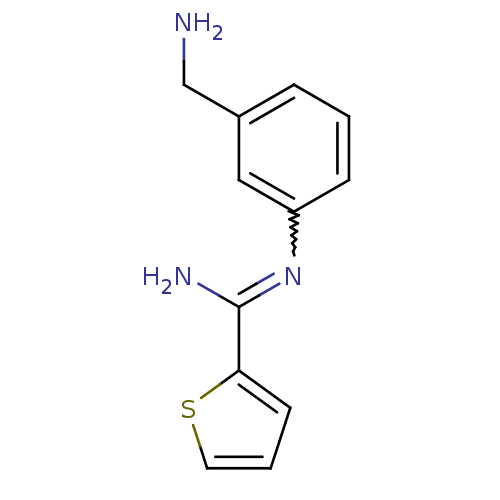
(CHEMBL553081 | CHEMBL555715 | N-(3-Aminomethyl-phe...)Show InChI InChI=1S/C12H13N3S/c13-8-9-3-1-4-10(7-9)15-12(14)11-5-2-6-16-11/h1-7H,8,13H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50065813

(CHEMBL555584 | N-(3-Aminomethyl-phenyl)-2-fluoro-a...)Show InChI InChI=1S/C9H12FN3/c10-5-9(12)13-8-3-1-2-7(4-8)6-11/h1-4H,5-6,11H2,(H2,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50065823
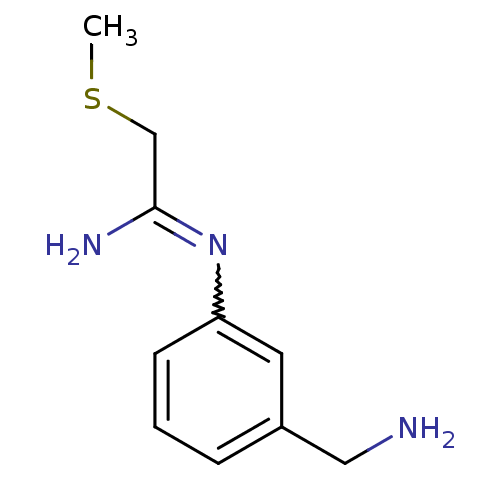
(CHEMBL555794 | N-(3-Aminomethyl-phenyl)-2-methylsu...)Show InChI InChI=1S/C10H15N3S/c1-14-7-10(12)13-9-4-2-3-8(5-9)6-11/h2-5H,6-7,11H2,1H3,(H2,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50225106

((2S)-2-amino-5-{[(E)-amino(nitroimino)methyl]amino...)Show SMILES N[C@@H](CCCNC(N)=N[N+]([O-])=O)C(O)=O |r,w:8.8| Show InChI InChI=1S/C6H13N5O4/c7-4(5(12)13)2-1-3-9-6(8)10-11(14)15/h4H,1-3,7H2,(H,12,13)(H3,8,9,10)/t4-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50065823

(CHEMBL555794 | N-(3-Aminomethyl-phenyl)-2-methylsu...)Show InChI InChI=1S/C10H15N3S/c1-14-7-10(12)13-9-4-2-3-8(5-9)6-11/h2-5H,6-7,11H2,1H3,(H2,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human inducible nitric oxide synthase (iNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM85530
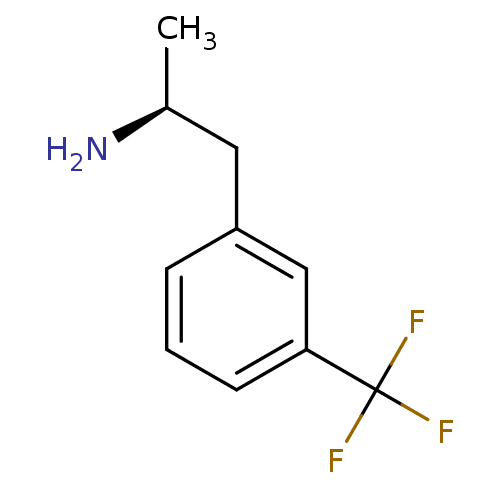
(Nor-d-fenfluramine | Nor-dexfenfluramine | Norfenf...)Show InChI InChI=1S/C10H12F3N/c1-7(14)5-8-3-2-4-9(6-8)10(11,12)13/h2-4,6-7H,5,14H2,1H3/t7-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 75-81 (2000)
BindingDB Entry DOI: 10.7270/Q2P26WPH |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50225106

((2S)-2-amino-5-{[(E)-amino(nitroimino)methyl]amino...)Show SMILES N[C@@H](CCCNC(N)=N[N+]([O-])=O)C(O)=O |r,w:8.8| Show InChI InChI=1S/C6H13N5O4/c7-4(5(12)13)2-1-3-9-6(8)10-11(14)15/h4H,1-3,7H2,(H,12,13)(H3,8,9,10)/t4-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human endothelial nitric oxide synthase (eNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50065833
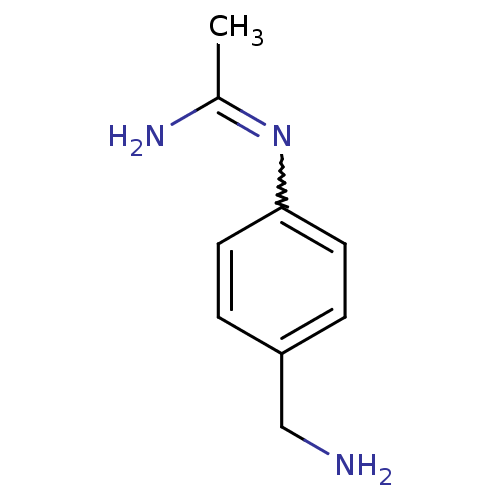
(CHEMBL542432 | N-(4-Aminomethyl-phenyl)-acetamidin...)Show InChI InChI=1S/C9H13N3/c1-7(11)12-9-4-2-8(6-10)3-5-9/h2-5H,6,10H2,1H3,(H2,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50065842
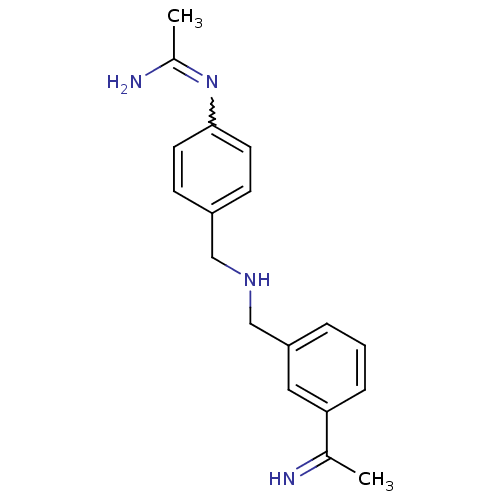
(CHEMBL540048 | N-(4-{[3-(1-Imino-ethyl)-benzylamin...)Show SMILES CC(N)=Nc1ccc(CNCc2cccc(c2)C(C)=N)cc1 |w:3.3| Show InChI InChI=1S/C18H22N4/c1-13(19)17-5-3-4-16(10-17)12-21-11-15-6-8-18(9-7-15)22-14(2)20/h3-10,19,21H,11-12H2,1-2H3,(H2,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50065848
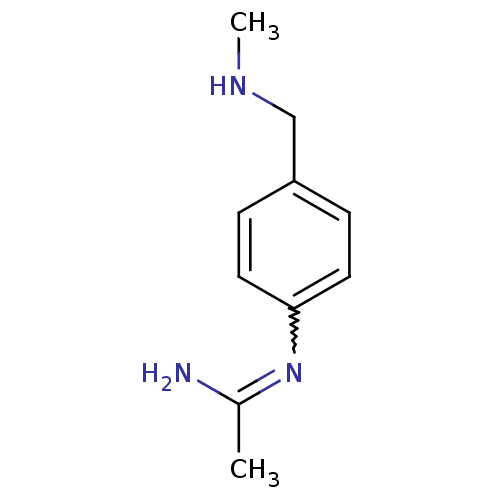
(CHEMBL539793 | N-(4-Methylaminomethyl-phenyl)-acet...)Show InChI InChI=1S/C10H15N3/c1-8(11)13-10-5-3-9(4-6-10)7-12-2/h3-6,12H,7H2,1-2H3,(H2,11,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM85530

(Nor-d-fenfluramine | Nor-dexfenfluramine | Norfenf...)Show InChI InChI=1S/C10H12F3N/c1-7(14)5-8-3-2-4-9(6-8)10(11,12)13/h2-4,6-7H,5,14H2,1H3/t7-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 75-81 (2000)
BindingDB Entry DOI: 10.7270/Q2P26WPH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM85598
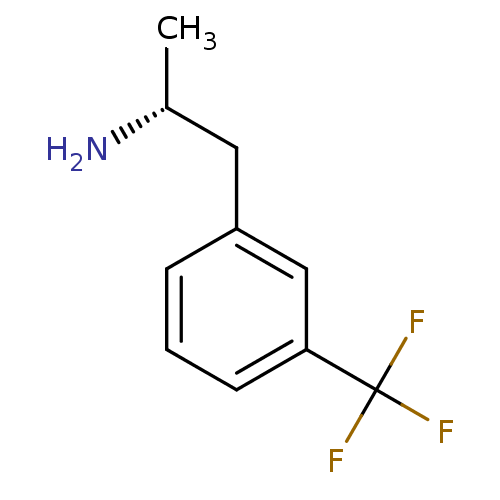
(l-norfenfluramine)Show InChI InChI=1S/C10H12F3N/c1-7(14)5-8-3-2-4-9(6-8)10(11,12)13/h2-4,6-7H,5,14H2,1H3/t7-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 75-81 (2000)
BindingDB Entry DOI: 10.7270/Q2P26WPH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM85598

(l-norfenfluramine)Show InChI InChI=1S/C10H12F3N/c1-7(14)5-8-3-2-4-9(6-8)10(11,12)13/h2-4,6-7H,5,14H2,1H3/t7-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 75-81 (2000)
BindingDB Entry DOI: 10.7270/Q2P26WPH |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50065843

(CHEMBL553081 | CHEMBL555715 | N-(3-Aminomethyl-phe...)Show InChI InChI=1S/C12H13N3S/c13-8-9-3-1-4-10(7-9)15-12(14)11-5-2-6-16-11/h1-7H,8,13H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human inducible nitric oxide synthase (iNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50065805
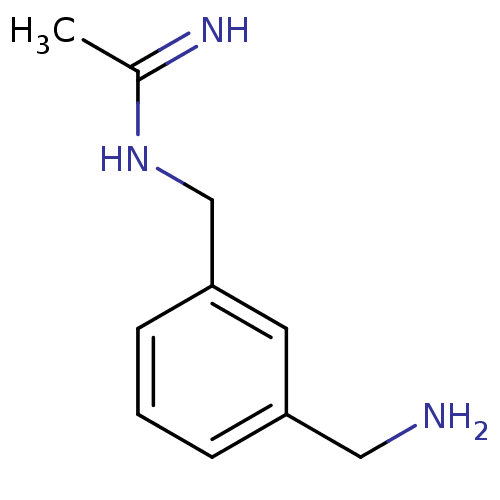
(CHEMBL544788 | N-(3-Aminomethyl-benzyl)-acetamidin...)Show InChI InChI=1S/C10H15N3/c1-8(12)13-7-10-4-2-3-9(5-10)6-11/h2-5H,6-7,11H2,1H3,(H2,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human inducible nitric oxide synthase (iNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50065807

(CHEMBL552825 | N-(3-Aminomethyl-phenyl)-furan-2-ca...)Show InChI InChI=1S/C12H13N3O/c13-8-9-3-1-4-10(7-9)15-12(14)11-5-2-6-16-11/h1-7H,8,13H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human inducible nitric oxide synthase (iNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50065818
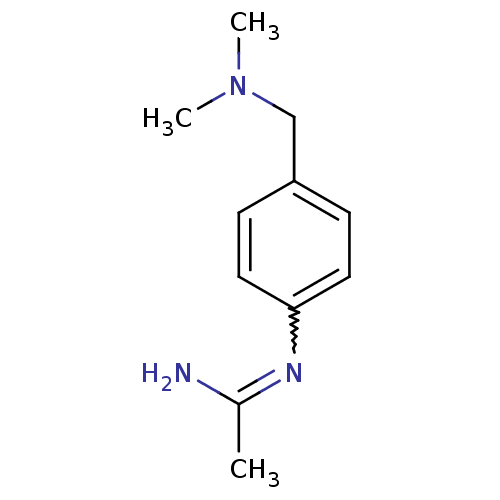
(CHEMBL554201 | N-(4-Dimethylaminomethyl-phenyl)-ac...)Show InChI InChI=1S/C11H17N3/c1-9(12)13-11-6-4-10(5-7-11)8-14(2)3/h4-7H,8H2,1-3H3,(H2,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13465

((2S)-N-[(1S)-1-carbamoyl-2-{4-[(5S)-1,1,3-trioxo-1...)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccc(cc1)[C@@H]1CC(=O)NS1(=O)=O)C(N)=O |r| Show InChI InChI=1S/C23H26N4O6S/c1-14(28)25-19(12-15-5-3-2-4-6-15)23(31)26-18(22(24)30)11-16-7-9-17(10-8-16)20-13-21(29)27-34(20,32)33/h2-10,18-20H,11-13H2,1H3,(H2,24,30)(H,25,28)(H,26,31)(H,27,29)/t18-,19-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition constant against protein-tyrosine phosphatase 1B by PNPP enzyme assay |
J Med Chem 48: 6544-8 (2005)
Article DOI: 10.1021/jm0504555
BindingDB Entry DOI: 10.7270/Q2805252 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM85530

(Nor-d-fenfluramine | Nor-dexfenfluramine | Norfenf...)Show InChI InChI=1S/C10H12F3N/c1-7(14)5-8-3-2-4-9(6-8)10(11,12)13/h2-4,6-7H,5,14H2,1H3/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 187 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 75-81 (2000)
BindingDB Entry DOI: 10.7270/Q2P26WPH |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50065817
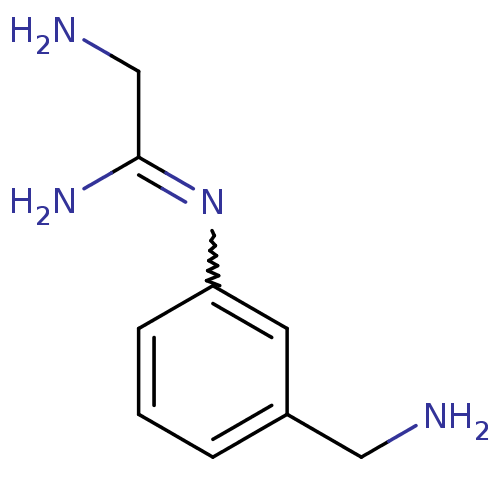
(2-Amino-N-(3-aminomethyl-phenyl)-acetamidine | CHE...)Show InChI InChI=1S/C9H14N4/c10-5-7-2-1-3-8(4-7)13-9(12)6-11/h1-4H,5-6,10-11H2,(H2,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM85598

(l-norfenfluramine)Show InChI InChI=1S/C10H12F3N/c1-7(14)5-8-3-2-4-9(6-8)10(11,12)13/h2-4,6-7H,5,14H2,1H3/t7-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 267 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 75-81 (2000)
BindingDB Entry DOI: 10.7270/Q2P26WPH |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50065847
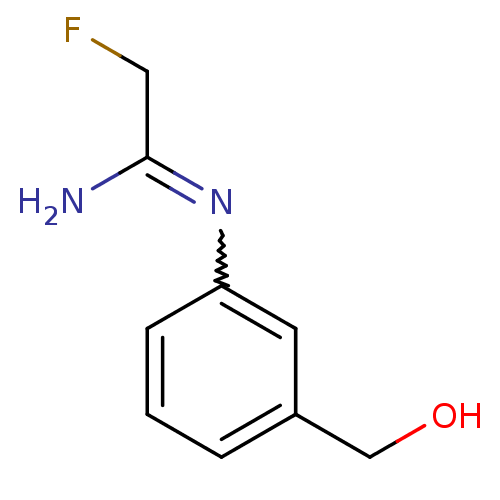
(2-Fluoro-N-(3-hydroxymethyl-phenyl)-acetamidine | ...)Show InChI InChI=1S/C9H11FN2O/c10-5-9(11)12-8-3-1-2-7(4-8)6-13/h1-4,13H,5-6H2,(H2,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50065823

(CHEMBL555794 | N-(3-Aminomethyl-phenyl)-2-methylsu...)Show InChI InChI=1S/C10H15N3S/c1-14-7-10(12)13-9-4-2-3-8(5-9)6-11/h2-5H,6-7,11H2,1H3,(H2,12,13) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human endothelial nitric oxide synthase (eNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50065812
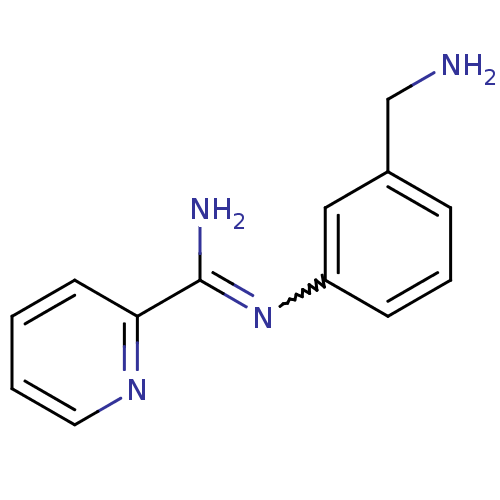
(CHEMBL544793 | N-(3-Aminomethyl-phenyl)-pyridine-2...)Show InChI InChI=1S/C13H14N4/c14-9-10-4-3-5-11(8-10)17-13(15)12-6-1-2-7-16-12/h1-8H,9,14H2,(H2,15,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50065807

(CHEMBL552825 | N-(3-Aminomethyl-phenyl)-furan-2-ca...)Show InChI InChI=1S/C12H13N3O/c13-8-9-3-1-4-10(7-9)15-12(14)11-5-2-6-16-11/h1-7H,8,13H2,(H2,14,15) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human endothelial nitric oxide synthase (eNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50065809
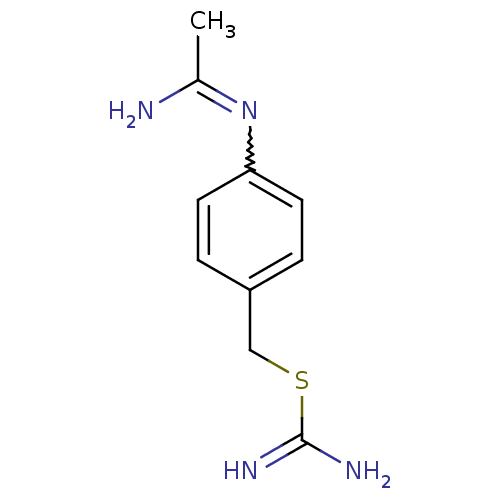
(CHEMBL544317 | N-(4-Carbamimidoylsulfanylmethyl-ph...)Show InChI InChI=1S/C10H14N4S/c1-7(11)14-9-4-2-8(3-5-9)6-15-10(12)13/h2-5H,6H2,1H3,(H2,11,14)(H3,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50065841
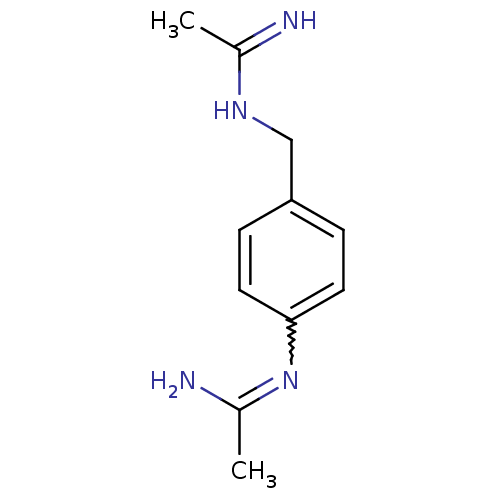
(CHEMBL545025 | N-[4-(Acetimidoylamino-methyl)-phen...)Show InChI InChI=1S/C11H16N4/c1-8(12)14-7-10-3-5-11(6-4-10)15-9(2)13/h3-6H,7H2,1-2H3,(H2,12,14)(H2,13,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50065843

(CHEMBL553081 | CHEMBL555715 | N-(3-Aminomethyl-phe...)Show InChI InChI=1S/C12H13N3S/c13-8-9-3-1-4-10(7-9)15-12(14)11-5-2-6-16-11/h1-7H,8,13H2,(H2,14,15) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human endothelial nitric oxide synthase (eNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50230993
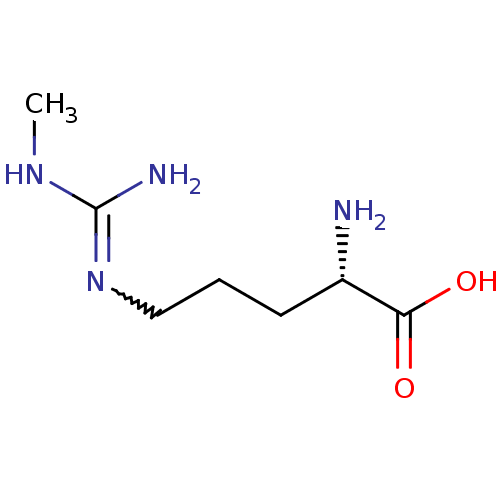
((2S)-2-amino-5-[(N-methylcarbamimidoyl)amino]penta...)Show InChI InChI=1S/C7H16N4O2/c1-10-7(9)11-4-2-3-5(8)6(12)13/h5H,2-4,8H2,1H3,(H,12,13)(H3,9,10,11)/t5-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human endothelial nitric oxide synthase (eNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50065813

(CHEMBL555584 | N-(3-Aminomethyl-phenyl)-2-fluoro-a...)Show InChI InChI=1S/C9H12FN3/c10-5-9(12)13-8-3-1-2-7(4-8)6-11/h1-4H,5-6,11H2,(H2,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human inducible nitric oxide synthase (iNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50065815

(4-Acetimidoylamino-benzamidine | CHEMBL543380)Show InChI InChI=1S/C9H12N4/c1-6(10)13-8-4-2-7(3-5-8)9(11)12/h2-5H,1H3,(H2,10,13)(H3,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50065803
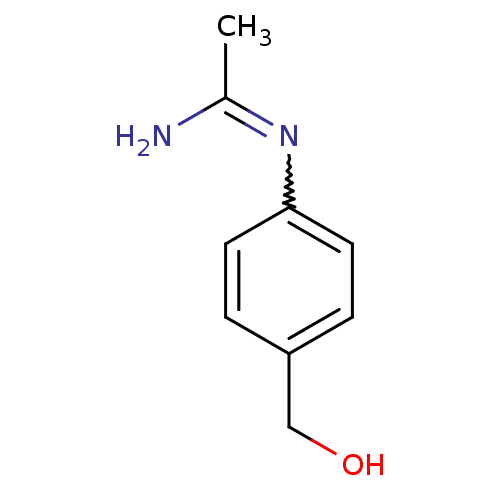
(CHEMBL542431 | N-(4-Hydroxymethyl-phenyl)-acetamid...)Show InChI InChI=1S/C9H12N2O/c1-7(10)11-9-4-2-8(6-12)3-5-9/h2-5,12H,6H2,1H3,(H2,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50225106

((2S)-2-amino-5-{[(E)-amino(nitroimino)methyl]amino...)Show SMILES N[C@@H](CCCNC(N)=N[N+]([O-])=O)C(O)=O |r,w:8.8| Show InChI InChI=1S/C6H13N5O4/c7-4(5(12)13)2-1-3-9-6(8)10-11(14)15/h4H,1-3,7H2,(H,12,13)(H3,8,9,10)/t4-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human inducible nitric oxide synthase (iNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM85597
(CAS_37577-24-5 | NSC_65801 | l-Fenfluramine)Show InChI InChI=1S/C12H16F3N/c1-3-16-9(2)7-10-5-4-6-11(8-10)12(13,14)15/h4-6,8-9,16H,3,7H2,1-2H3/t9-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 75-81 (2000)
BindingDB Entry DOI: 10.7270/Q2P26WPH |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50230993

((2S)-2-amino-5-[(N-methylcarbamimidoyl)amino]penta...)Show InChI InChI=1S/C7H16N4O2/c1-10-7(9)11-4-2-3-5(8)6(12)13/h5H,2-4,8H2,1H3,(H,12,13)(H3,9,10,11)/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50230993

((2S)-2-amino-5-[(N-methylcarbamimidoyl)amino]penta...)Show InChI InChI=1S/C7H16N4O2/c1-10-7(9)11-4-2-3-5(8)6(12)13/h5H,2-4,8H2,1H3,(H,12,13)(H3,9,10,11)/t5-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human inducible nitric oxide synthase (iNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50065838
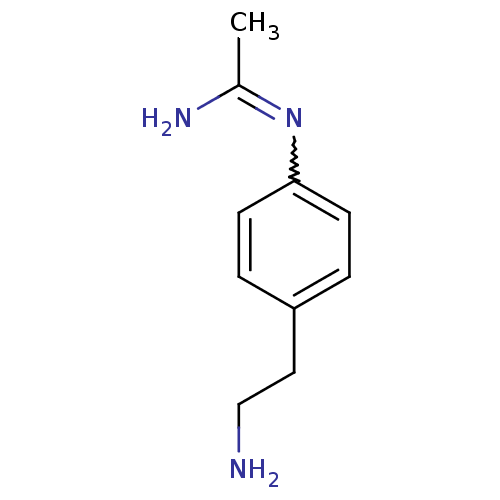
(CHEMBL538767 | N-[4-(2-Amino-ethyl)-phenyl]-acetam...)Show InChI InChI=1S/C10H15N3/c1-8(12)13-10-4-2-9(3-5-10)6-7-11/h2-5H,6-7,11H2,1H3,(H2,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50300312
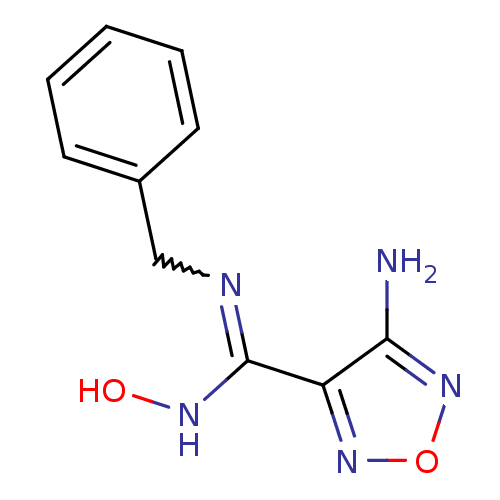
(4-amino-1,2,5-oxadiazole-3-carboximidamide | CHEMB...)Show InChI InChI=1S/C10H11N5O2/c11-9-8(14-17-15-9)10(13-16)12-6-7-4-2-1-3-5-7/h1-5,16H,6H2,(H2,11,15)(H,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal his-tagged human indoleamine 2,3-dioxygenase expressed in Escherichia coli assessed as N'-formylkynurenine formation by spec... |
J Med Chem 52: 7364-7 (2009)
Article DOI: 10.1021/jm900518f
BindingDB Entry DOI: 10.7270/Q29P32KW |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50065813

(CHEMBL555584 | N-(3-Aminomethyl-phenyl)-2-fluoro-a...)Show InChI InChI=1S/C9H12FN3/c10-5-9(12)13-8-3-1-2-7(4-8)6-11/h1-4H,5-6,11H2,(H2,12,13) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human endothelial nitric oxide synthase (eNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50065826
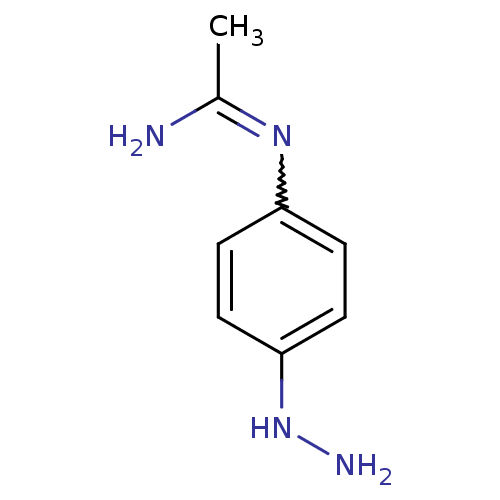
(CHEMBL543142 | N-(4-Hydrazino-phenyl)-acetamidine)Show InChI InChI=1S/C8H12N4/c1-6(9)11-7-2-4-8(12-10)5-3-7/h2-5,12H,10H2,1H3,(H2,9,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM85597

(CAS_37577-24-5 | NSC_65801 | l-Fenfluramine)Show InChI InChI=1S/C12H16F3N/c1-3-16-9(2)7-10-5-4-6-11(8-10)12(13,14)15/h4-6,8-9,16H,3,7H2,1-2H3/t9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 75-81 (2000)
BindingDB Entry DOI: 10.7270/Q2P26WPH |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50065828
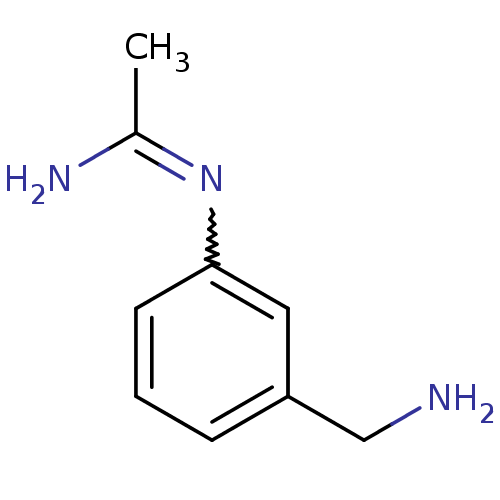
(CHEMBL542678 | N-(3-Aminomethyl-phenyl)-acetamidin...)Show InChI InChI=1S/C9H13N3/c1-7(11)12-9-4-2-3-8(5-9)6-10/h2-5H,6,10H2,1H3,(H2,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50065824
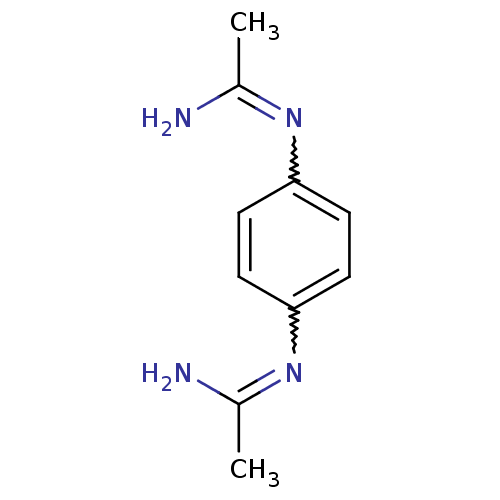
(CHEMBL544090 | N-(4-Acetimidoylamino-phenyl)-aceta...)Show InChI InChI=1S/C10H14N4/c1-7(11)13-9-3-5-10(6-4-9)14-8(2)12/h3-6H,1-2H3,(H2,11,13)(H2,12,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50065842

(CHEMBL540048 | N-(4-{[3-(1-Imino-ethyl)-benzylamin...)Show SMILES CC(N)=Nc1ccc(CNCc2cccc(c2)C(C)=N)cc1 |w:3.3| Show InChI InChI=1S/C18H22N4/c1-13(19)17-5-3-4-16(10-17)12-21-11-15-6-8-18(9-7-15)22-14(2)20/h3-10,19,21H,11-12H2,1-2H3,(H2,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human inducible nitric oxide synthase (iNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM85597

(CAS_37577-24-5 | NSC_65801 | l-Fenfluramine)Show InChI InChI=1S/C12H16F3N/c1-3-16-9(2)7-10-5-4-6-11(8-10)12(13,14)15/h4-6,8-9,16H,3,7H2,1-2H3/t9-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 75-81 (2000)
BindingDB Entry DOI: 10.7270/Q2P26WPH |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50065848

(CHEMBL539793 | N-(4-Methylaminomethyl-phenyl)-acet...)Show InChI InChI=1S/C10H15N3/c1-8(11)13-10-5-3-9(4-6-10)7-12-2/h3-6,12H,7H2,1-2H3,(H2,11,13) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human endothelial nitric oxide synthase (eNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50065833

(CHEMBL542432 | N-(4-Aminomethyl-phenyl)-acetamidin...)Show InChI InChI=1S/C9H13N3/c1-7(11)12-9-4-2-8(6-10)3-5-9/h2-5H,6,10H2,1H3,(H2,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human inducible nitric oxide synthase (iNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50065841

(CHEMBL545025 | N-[4-(Acetimidoylamino-methyl)-phen...)Show InChI InChI=1S/C11H16N4/c1-8(12)14-7-10-3-5-11(6-4-10)15-9(2)13/h3-6H,7H2,1-2H3,(H2,12,14)(H2,13,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human inducible nitric oxide synthase (iNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50065805

(CHEMBL544788 | N-(3-Aminomethyl-benzyl)-acetamidin...)Show InChI InChI=1S/C10H15N3/c1-8(12)13-7-10-4-2-3-9(5-10)6-11/h2-5H,6-7,11H2,1H3,(H2,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data