| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cholecystokinin receptor type A |
|---|
| Ligand | BDBM50011542 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_50182 |
|---|
| IC50 | 26±n/a nM |
|---|
| Citation |  Tilley, JW; Danho, W; Lovey, K; Wagner, R; Swistok, J; Makofske, R; Michalewsky, J; Triscari, J; Nelson, D; Weatherford, S Carboxylic acids and tetrazoles as isosteric replacements for sulfate in cholecystokinin analogues. J Med Chem34:1125-36 (1991) [PubMed] Tilley, JW; Danho, W; Lovey, K; Wagner, R; Swistok, J; Makofske, R; Michalewsky, J; Triscari, J; Nelson, D; Weatherford, S Carboxylic acids and tetrazoles as isosteric replacements for sulfate in cholecystokinin analogues. J Med Chem34:1125-36 (1991) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cholecystokinin receptor type A |
|---|
| Name: | Cholecystokinin receptor type A |
|---|
| Synonyms: | CCKAR_RAT | Cckar | Cholecystokinin peripheral | Cholecystokinin receptor | Cholecystokinin receptor type A |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 49676.37 |
|---|
| Organism: | RAT |
|---|
| Description: | Cholecystokinin central 0 RAT::P30551 |
|---|
| Residue: | 444 |
|---|
| Sequence: | MSHSPARQHLVESSRMDVVDSLLMNGSNITPPCELGLENETLFCLDQPQPSKEWQSALQI
LLYSIIFLLSVLGNTLVITVLIRNKRMRTVTNIFLLSLAVSDLMLCLFCMPFNLIPNLLK
DFIFGSAVCKTTTYFMGTSVSVSTFNLVAISLERYGAICRPLQSRVWQTKSHALKVIAAT
WCLSFTIMTPYPIYSNLVPFTKNNNQTANMCRFLLPSDAMQQSWQTFLLLILFLLPGIVM
VVAYGLISLELYQGIKFDASQKKSAKEKKPSTGSSTRYEDSDGCYLQKSRPPRKLELQQL
SSGSGGSRLNRIRSSSSAANLIAKKRVIRMLIVIVVLFFLCWMPIFSANAWRAYDTVSAE
KHLSGTPISFILLLSYTSSCVNPIIYCFMNKRFRLGFMATFPCCPNPGPPGVRGEVGEEE
DGRTIRALLSRYSYSHMSTSAPPP
|
|
|
|---|
| BDBM50011542 |
|---|
| n/a |
|---|
| Name | BDBM50011542 |
|---|
| Synonyms: | 3-[4-(carboxymethyl)phenyl]propanoyl-Met-Gly-Trp-Met-Asp-Phe-NH2 | CHEMBL273344 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C47H58N8O11S2 |
|---|
| Mol. Mass. | 975.14 |
|---|
| SMILES | CSCC[C@H](NC(=O)CCc1ccc(CC(O)=O)cc1)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |
|---|
| Structure | 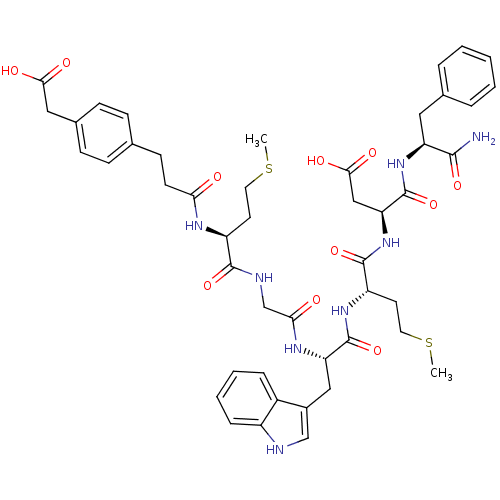
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Tilley, JW; Danho, W; Lovey, K; Wagner, R; Swistok, J; Makofske, R; Michalewsky, J; Triscari, J; Nelson, D; Weatherford, S Carboxylic acids and tetrazoles as isosteric replacements for sulfate in cholecystokinin analogues. J Med Chem34:1125-36 (1991) [PubMed]
Tilley, JW; Danho, W; Lovey, K; Wagner, R; Swistok, J; Makofske, R; Michalewsky, J; Triscari, J; Nelson, D; Weatherford, S Carboxylic acids and tetrazoles as isosteric replacements for sulfate in cholecystokinin analogues. J Med Chem34:1125-36 (1991) [PubMed]