

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50068612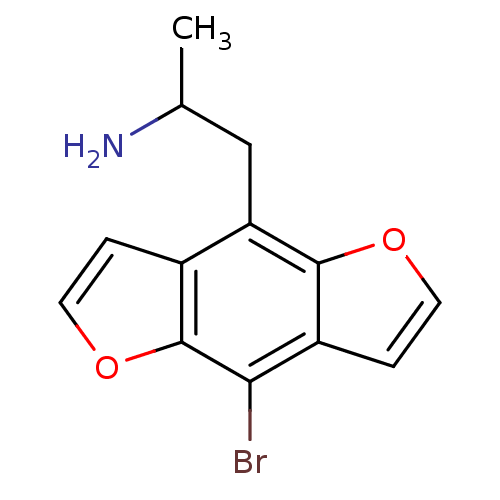 (2-(8-Bromo-benzo[1,2-b;4,5-b']difuran-4-yl)-1-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding activity against cloned human 5-hydroxytryptamine 2C receptor using [125I]-DOI as the radioligand. | J Med Chem 41: 5148-9 (1999) Article DOI: 10.1021/jm9803525 BindingDB Entry DOI: 10.7270/Q2862FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50068612 (2-(8-Bromo-benzo[1,2-b;4,5-b']difuran-4-yl)-1-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding activity against cloned human 5-hydroxytryptamine 2A receptor using [125I]-DOI as the radioligand. | J Med Chem 41: 5148-9 (1999) Article DOI: 10.1021/jm9803525 BindingDB Entry DOI: 10.7270/Q2862FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50130172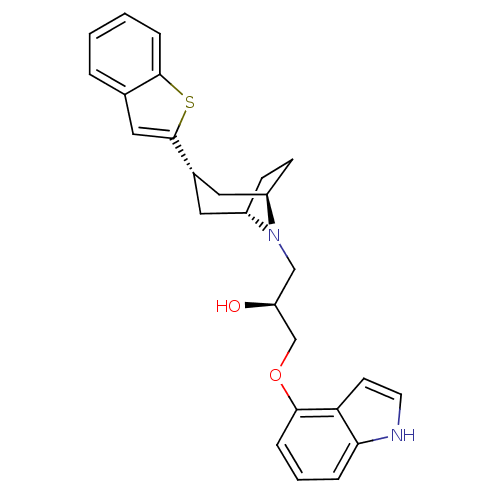 (1-(3-Benzo[b]thiophen-2-yl-8-aza-bicyclo[3.2.1]oct...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro affinity of the compound at the 5-HT reuptake site using [3H]-paroxetine as radioligand in rat frontal cortex membranes | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50014997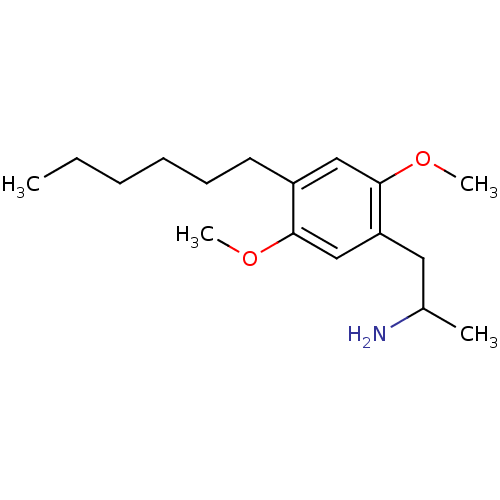 (1-(4-hexyl-2,5-dimethoxyphenyl)propan-2-amine | 2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by PDSP Ki Database | Naunyn Schmiedebergs Arch Pharmacol 359: 1-6 (1999) Article DOI: 10.1007/pl00005315 BindingDB Entry DOI: 10.7270/Q2862F09 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM86231 (ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by PDSP Ki Database | Schizophr Res 37: 107-22 (1999) Article DOI: 10.1016/s0920-9964(98)00146-7 BindingDB Entry DOI: 10.7270/Q23F4N5N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50064340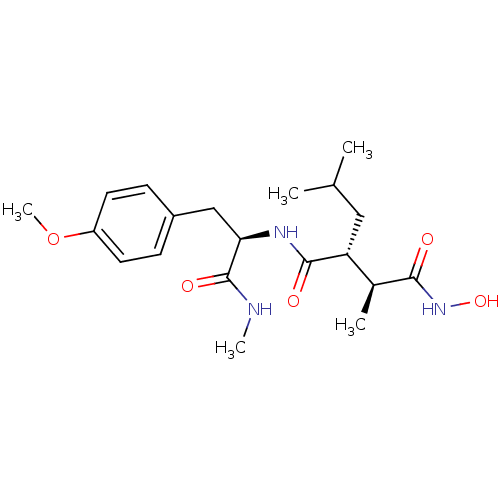 ((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-[(R)-2-(4-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of MMP-9 (Matrix metalloproteinase-9) | J Med Chem 41: 1745-8 (1998) Article DOI: 10.1021/jm970849z BindingDB Entry DOI: 10.7270/Q2GB235N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM139889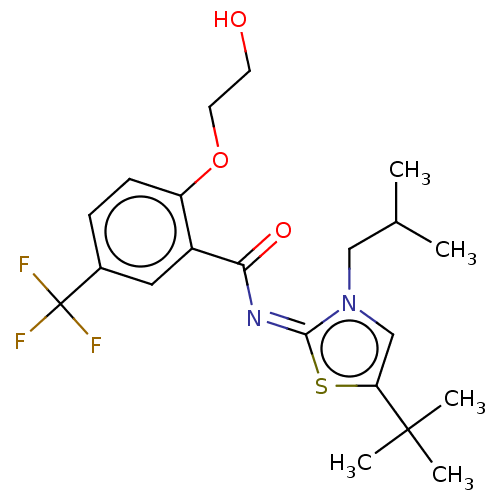 (US8895592, 19) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The CB1 and CB2 radioligand binding assays described herein are utilized to ascertain the selectivity of compounds of the present application for bin... | US Patent US8895592 (2014) BindingDB Entry DOI: 10.7270/Q2CV4GDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50130170 ((S)-1-(1H-Indol-4-yloxy)-3-[(1R,3S,5S)-3-(4-methox...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity at 5-hydroxytryptamine 1A receptor by [3H]-8-OH-DPAT displacement. | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50130170 ((S)-1-(1H-Indol-4-yloxy)-3-[(1R,3S,5S)-3-(4-methox...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro affinity of the compound at the 5-HT reuptake site using [3H]-paroxetine as radioligand in rat frontal cortex membranes | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50135254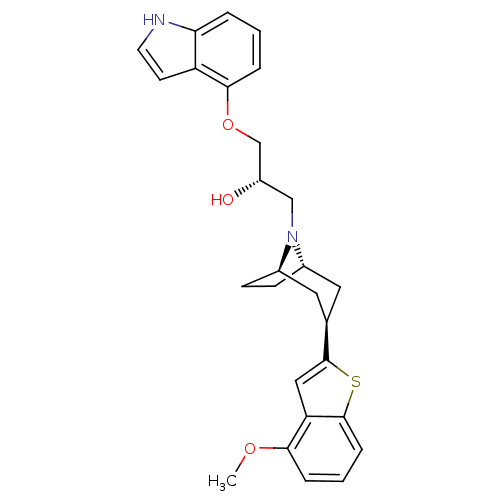 ((S)-1-(1H-Indol-4-yloxy)-3-[(1S,3R,5R)-3-(4-methox...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Affinity at the 5-HT reuptake site labeled with [3H]-paroxetine using rat frontal cortex membranes | Bioorg Med Chem Lett 13: 3939-42 (2003) BindingDB Entry DOI: 10.7270/Q24T6HSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50068612 (2-(8-Bromo-benzo[1,2-b;4,5-b']difuran-4-yl)-1-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding activity against cloned human 5-hydroxytryptamine 2B receptor using [3H]-5-HT as the radioligand. | J Med Chem 41: 5148-9 (1999) Article DOI: 10.1021/jm9803525 BindingDB Entry DOI: 10.7270/Q2862FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50068612 (2-(8-Bromo-benzo[1,2-b;4,5-b']difuran-4-yl)-1-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Antagonistic activity measured for 5-hydroxytryptamine 2A receptor using [3H]-MDL- 100,907 as radioligand in rat cortical homogenates. | J Med Chem 41: 5148-9 (1999) Article DOI: 10.1021/jm9803525 BindingDB Entry DOI: 10.7270/Q2862FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50135249 ((S)-1-((2S,4R)-4-(5-fluorobenzo[b]thiophen-2-yl)-2...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Affinity at the 5-HT reuptake site labeled with [3H]-paroxetine using rat frontal cortex membranes | Bioorg Med Chem Lett 13: 3939-42 (2003) BindingDB Entry DOI: 10.7270/Q24T6HSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50076428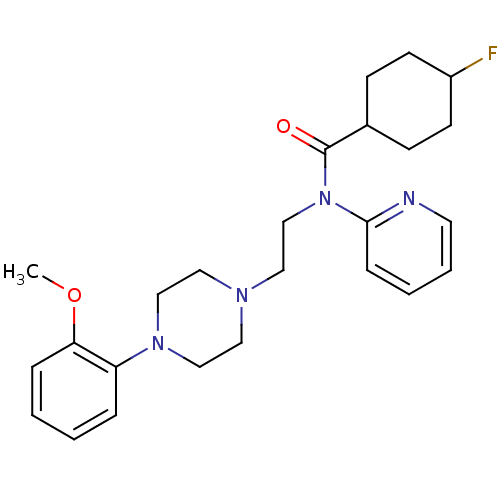 (4-Fluoro-cyclohexanecarboxylic acid {2-[4-(2-metho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.247 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description In vitro inhibition of [3H]- 8-OH-DPAT binding to cloned cell line containing human 5-hydroxytryptamine 1A receptor by Panlabs assay | J Med Chem 42: 1576-86 (1999) Article DOI: 10.1021/jm980456f BindingDB Entry DOI: 10.7270/Q23R0S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50130163 ((S)-1-(1H-Indol-4-yloxy)-3-[(4S,6R)-4-(4-methoxy-b...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro affinity of the compound at the 5-HT reuptake site using [3H]-paroxetine as radioligand in rat frontal cortex membranes | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM135877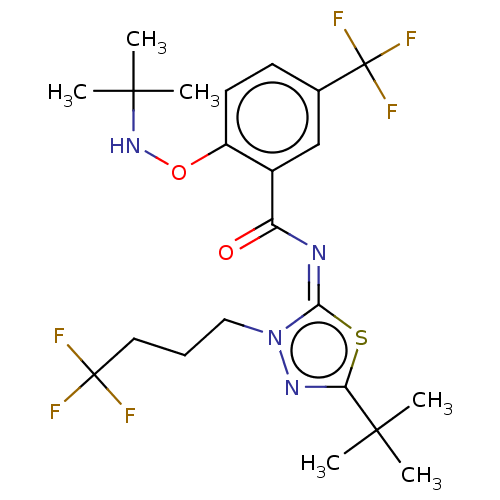 (US8859596, 164) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
AbbVie Inc. US Patent | Assay Description HEK293 cells stably expressing rat CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogenized... | US Patent US8859596 (2014) BindingDB Entry DOI: 10.7270/Q2W66JHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50145598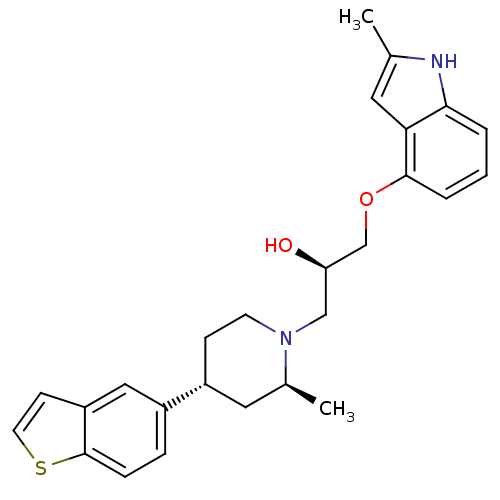 ((R)-1-((3S,4R)-4-Benzo[b]thiophen-5-yl-2-methyl-pi...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity at 5-HT reuptake site labeled with [3H]-paroxetine | Bioorg Med Chem Lett 14: 2653-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.088 BindingDB Entry DOI: 10.7270/Q2GF0SZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50052337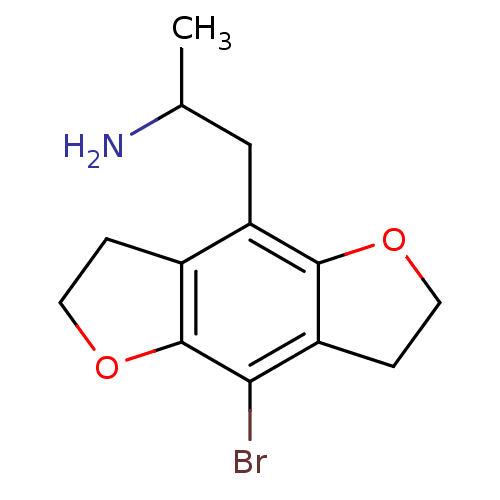 (2-(8-bromo-2,3,6,7-tetrahydro-benzo[1,2-b;4,5-b']d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Agonistic activity at cloned human 5-hydroxytryptamine 2C receptor using [125I]-DOI as radioligand | J Med Chem 39: 2953-61 (1996) Article DOI: 10.1021/jm960199j BindingDB Entry DOI: 10.7270/Q28914ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50064703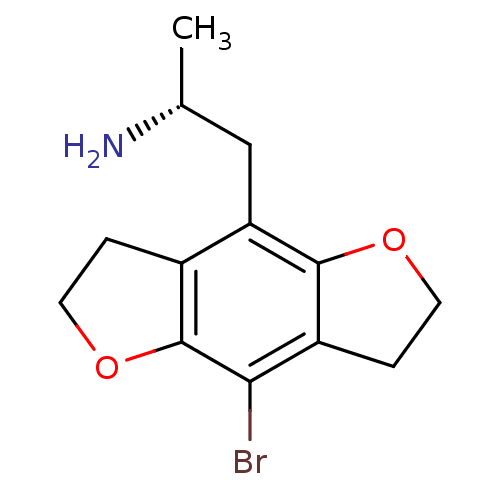 ((R)-2-(8-Bromo-2,3,6,7-tetrahydro-benzo[1,2-b;4,5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding ability of compound at cloned human 5-hydroxytryptamine 2C receptor using [125 I]DOI as radioligand | J Med Chem 41: 2134-45 (1998) Article DOI: 10.1021/jm980076u BindingDB Entry DOI: 10.7270/Q2XD10T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50052337 (2-(8-bromo-2,3,6,7-tetrahydro-benzo[1,2-b;4,5-b']d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding activity against cloned human 5-hydroxytryptamine 2C receptor using [125I]-DOI as the radioligand. | J Med Chem 41: 5148-9 (1999) Article DOI: 10.1021/jm9803525 BindingDB Entry DOI: 10.7270/Q2862FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50130152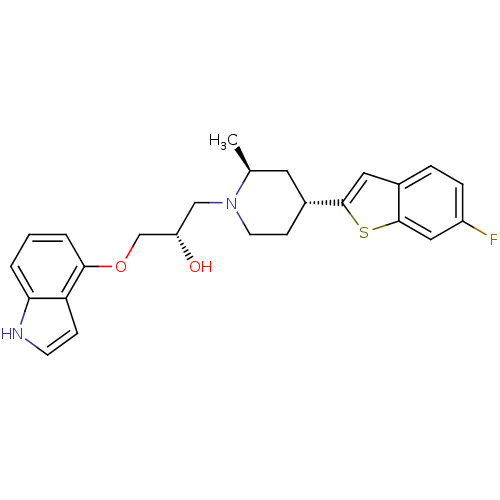 ((S)-1-(1H-indol-4-yloxy)-3-((2S,4R)-4-(6-fluoroben...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro affinity of the compound at the 5-HT reuptake site using [3H]-paroxetine as radioligand in rat frontal cortex membranes | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM135855 (US8859596, 141) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.320 | -55.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
AbbVie Inc. US Patent | Assay Description HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz... | US Patent US8859596 (2014) BindingDB Entry DOI: 10.7270/Q2W66JHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50452111 (CHEMBL2112353) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity at 5-HT reuptake site labeled with [3H]-paroxetine | Bioorg Med Chem Lett 14: 2653-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.088 BindingDB Entry DOI: 10.7270/Q2GF0SZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Mus musculus (Mouse)) | BDBM86708 (CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Receptor-linked G protein activation at 5-hydroxytryptamine receptor was determined by measuring the stimulation of [35S]-GTP-gammaS, binding (Experi... | J Med Chem 42: 1576-86 (1999) Article DOI: 10.1021/jm980456f BindingDB Entry DOI: 10.7270/Q23R0S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50135248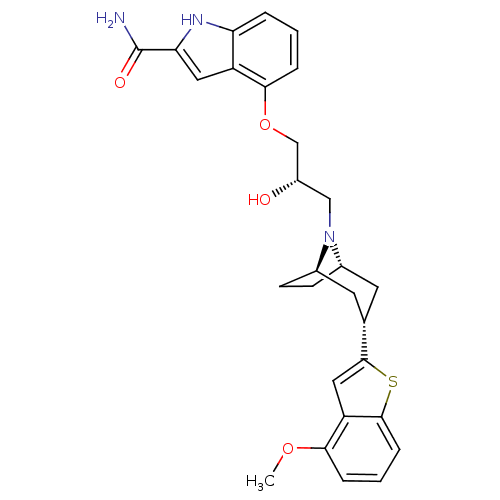 (4-{(S)-2-Hydroxy-3-[(1R,3S,5S)-3-(4-methoxy-benzo[...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Affinity at the 5-HT reuptake site labeled with [3H]-paroxetine using rat frontal cortex membranes | Bioorg Med Chem Lett 13: 3939-42 (2003) BindingDB Entry DOI: 10.7270/Q24T6HSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (PIG) | BDBM30704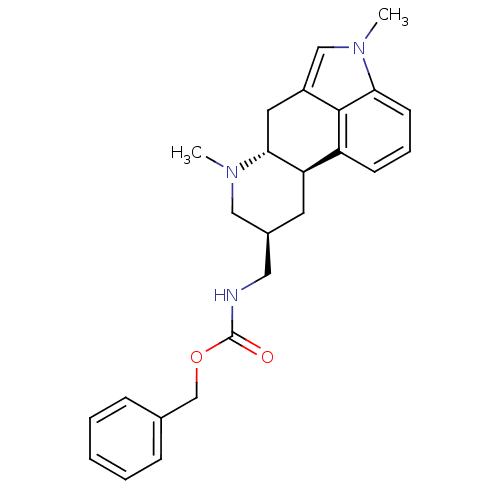 ((phenylmethyl) N-[[(6aR,9S,10aR)-4,7-dimethyl-6,6a...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by PDSP Ki Database | J Pharmacol Exp Ther 265: 1272-9 (1993) BindingDB Entry DOI: 10.7270/Q2833QJ4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50130173 ((S)-1-[(4S,6R)-4-(5-Fluoro-benzo[b]thiophen-2-yl)-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro affinity of the compound at the 5-HT reuptake site using [3H]-paroxetine as radioligand in rat frontal cortex membranes | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50135244 ((S)-1-[(2S,4R)-4-(6-Fluoro-benzo[b]thiophen-2-yl)-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Affinity at the 5-HT reuptake site labeled with [3H]-paroxetine using rat frontal cortex membranes | Bioorg Med Chem Lett 13: 3939-42 (2003) BindingDB Entry DOI: 10.7270/Q24T6HSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM135905 (US8859596, 193) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.400 | -54.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
AbbVie Inc. US Patent | Assay Description HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz... | US Patent US8859596 (2014) BindingDB Entry DOI: 10.7270/Q2W66JHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM135909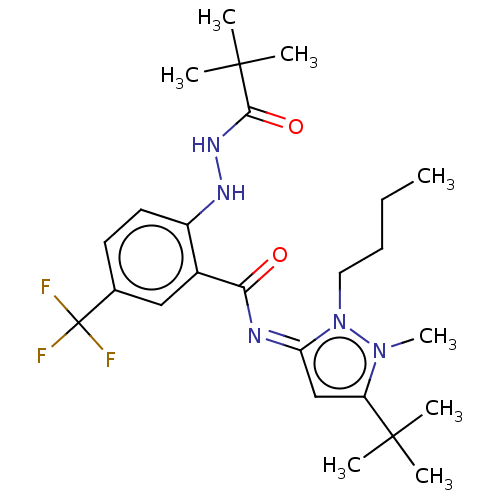 (US8859596, 198) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.400 | -54.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
AbbVie Inc. US Patent | Assay Description HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz... | US Patent US8859596 (2014) BindingDB Entry DOI: 10.7270/Q2W66JHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM86231 (ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by PDSP Ki Database | Schizophr Res 37: 107-22 (1999) Article DOI: 10.1016/s0920-9964(98)00146-7 BindingDB Entry DOI: 10.7270/Q23F4N5N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50452114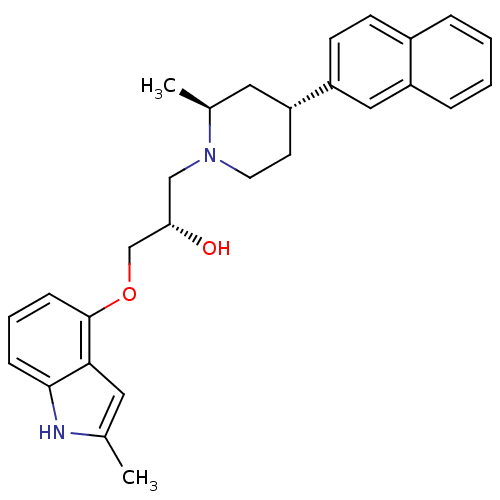 (CHEMBL2112350) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity at 5-HT reuptake site labeled with [3H]-paroxetine | Bioorg Med Chem Lett 14: 2653-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.088 BindingDB Entry DOI: 10.7270/Q2GF0SZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50001775 ((ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methyle...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by PDSP Ki Database | Naunyn Schmiedebergs Arch Pharmacol 357: 17-24 (1998) Article DOI: 10.1007/pl00005133 BindingDB Entry DOI: 10.7270/Q2W66J9P | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50019443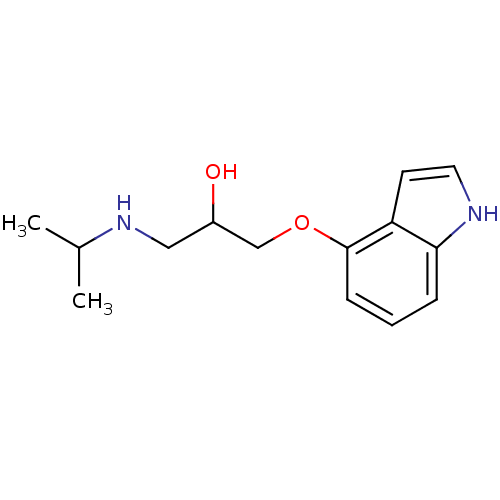 (1-(1H-Indol-4-yloxy)-3-isopropylamino-propan-2-ol ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity at human adrenergic beta2 receptor | Bioorg Med Chem Lett 17: 5600-4 (2007) Article DOI: 10.1016/j.bmcl.2007.07.086 BindingDB Entry DOI: 10.7270/Q2FJ2GG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50014999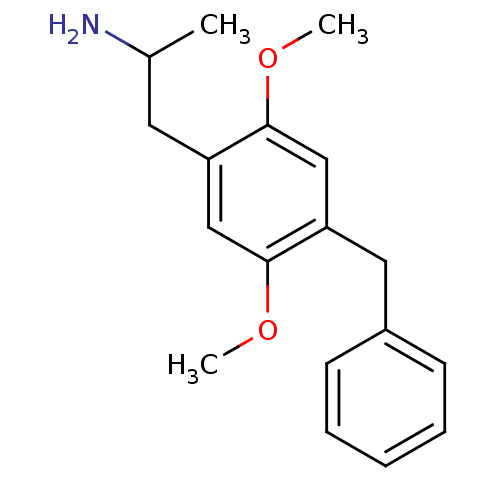 (CHEMBL274589 | DOBz) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by PDSP Ki Database | Naunyn Schmiedebergs Arch Pharmacol 359: 1-6 (1999) Article DOI: 10.1007/pl00005315 BindingDB Entry DOI: 10.7270/Q2862F09 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by PDSP Ki Database | J Pharmacol Exp Ther 276: 720-7 (1996) BindingDB Entry DOI: 10.7270/Q29W0D1S | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50130168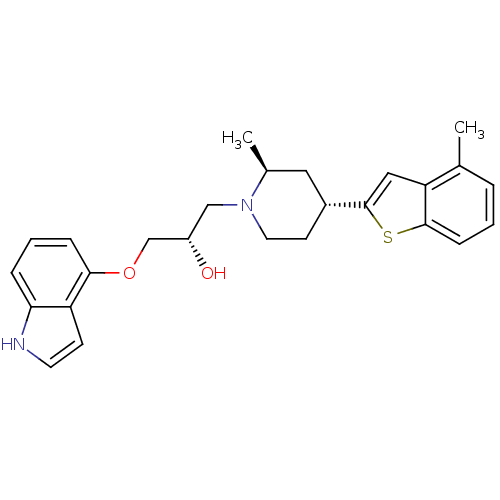 ((S)-1-(1H-Indol-4-yloxy)-3-[(4S,6R)-2-methyl-4-(4-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro affinity of the compound at the 5-HT reuptake site using [3H]-paroxetine as radioligand in rat frontal cortex membranes | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50135253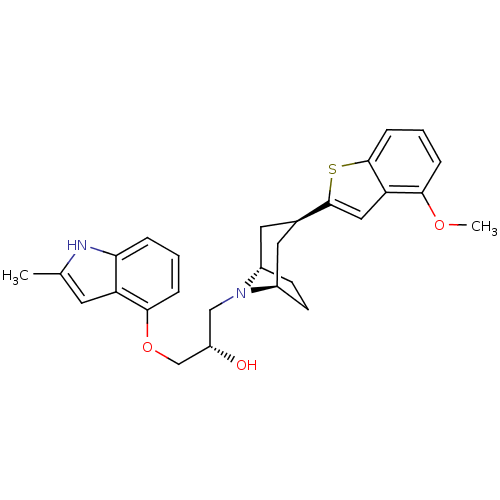 ((S)-1-[(1S,3R,5R)-3-(4-Methoxy-benzo[b]thiophen-2-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Affinity at the 5-HT reuptake site labeled with [3H]-paroxetine using rat frontal cortex membranes | Bioorg Med Chem Lett 13: 3939-42 (2003) BindingDB Entry DOI: 10.7270/Q24T6HSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50102594 (7-Isobutyl-8-oxo-2-oxa-9-aza-bicyclo[10.2.2]hexade...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibition of matrix metalloprotease-13 | J Med Chem 44: 2636-60 (2001) BindingDB Entry DOI: 10.7270/Q2FB53NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM28582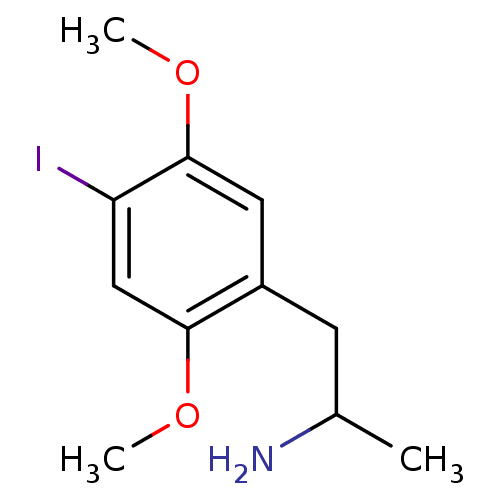 (1-(4-iodo-2,5-dimethoxyphenyl)propan-2-amine | CHE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding activity against cloned human 5-hydroxytryptamine 2A receptor using [125I]-DOI as the radioligand. | J Med Chem 41: 5148-9 (1999) Article DOI: 10.1021/jm9803525 BindingDB Entry DOI: 10.7270/Q2862FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50130269 ((6aR,9R)-5-Bromo-7-methyl-4,6,6a,7,8,9-hexahydro-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by PDSP Ki Database | J Pharmacol Exp Ther 276: 720-7 (1996) BindingDB Entry DOI: 10.7270/Q29W0D1S | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50052337 (2-(8-bromo-2,3,6,7-tetrahydro-benzo[1,2-b;4,5-b']d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Agonistic activity at cloned human 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand | J Med Chem 39: 2953-61 (1996) Article DOI: 10.1021/jm960199j BindingDB Entry DOI: 10.7270/Q28914ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50064703 ((R)-2-(8-Bromo-2,3,6,7-tetrahydro-benzo[1,2-b;4,5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding ability of compound at cloned human 5-hydroxytryptamine 2A receptor using [125 I]DOI as radioligand | J Med Chem 41: 2134-45 (1998) Article DOI: 10.1021/jm980076u BindingDB Entry DOI: 10.7270/Q2XD10T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50052337 (2-(8-bromo-2,3,6,7-tetrahydro-benzo[1,2-b;4,5-b']d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding activity against cloned human 5-hydroxytryptamine 2A receptor using [125I]-DOI as the radioligand. | J Med Chem 41: 5148-9 (1999) Article DOI: 10.1021/jm9803525 BindingDB Entry DOI: 10.7270/Q2862FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM135905 (US8859596, 193) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
AbbVie Inc. US Patent | Assay Description HEK293 cells stably expressing rat CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogenized... | US Patent US8859596 (2014) BindingDB Entry DOI: 10.7270/Q2W66JHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil collagenase (Homo sapiens (Human)) | BDBM50102594 (7-Isobutyl-8-oxo-2-oxa-9-aza-bicyclo[10.2.2]hexade...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibition of matrix metalloprotease-8 | J Med Chem 44: 2636-60 (2001) BindingDB Entry DOI: 10.7270/Q2FB53NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM84745 (CAS_136434-34-9 | DULOXETINE | LY-248686 | LY24868...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by PDSP Ki Database | Neuropsychopharmacology 25: 871-80 (2001) Article DOI: 10.1016/S0893-133X(01)00298-6 BindingDB Entry DOI: 10.7270/Q25M648W | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50130157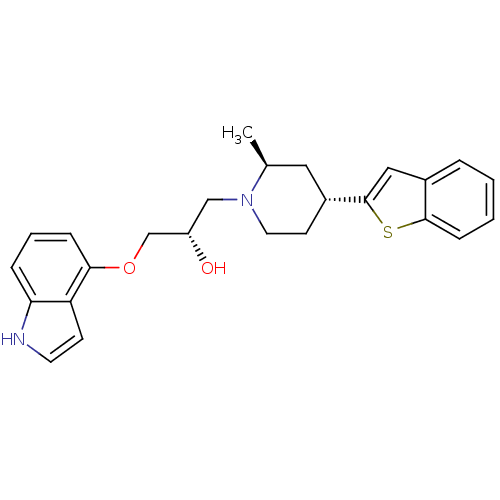 ((S)-1-((4S,6R)-4-Benzo[b]thiophen-2-yl-2-methyl-pi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro affinity of the compound at the 5-HT reuptake site using [3H]-paroxetine as radioligand in rat frontal cortex membranes | Bioorg Med Chem Lett 13: 2393-7 (2003) BindingDB Entry DOI: 10.7270/Q2Z31Z1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50076428 (4-Fluoro-cyclohexanecarboxylic acid {2-[4-(2-metho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.515 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Compound was tested in vitro for the inhibition of [3H]- 8-OH-DPAT binding to cloned cell line containing human 5-hydroxytryptamine 1A receptor | J Med Chem 42: 1576-86 (1999) Article DOI: 10.1021/jm980456f BindingDB Entry DOI: 10.7270/Q23R0S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50019443 (1-(1H-Indol-4-yloxy)-3-isopropylamino-propan-2-ol ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity at human adrenergic beta1 receptor | Bioorg Med Chem Lett 17: 5600-4 (2007) Article DOI: 10.1016/j.bmcl.2007.07.086 BindingDB Entry DOI: 10.7270/Q2FJ2GG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3944 total ) | Next | Last >> |