

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gastrin/cholecystokinin type B receptor (Bos taurus) | BDBM50003204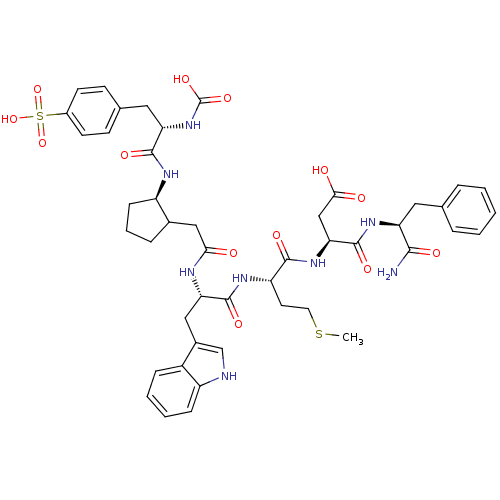 (CHEMBL122438 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of [3H]-propanoyl-CCK-8 binding to Cholecystokinin type B receptor in bovine striatum | J Med Chem 35: 3774-83 (1992) BindingDB Entry DOI: 10.7270/Q2CC0ZMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50003666 (3-[2-[2-tert-Butoxycarbonylamino-3-(1H-indol-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibitory activity against cholecystokinin type A receptor from rat pancreas binding assay | J Med Chem 35: 4249-52 (1992) BindingDB Entry DOI: 10.7270/Q2Z60N07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50003669 (Ac-Tyr(SO3H)-Met-Gly-Trp-Met-R-Dtc-Phe-NH2 | CHEMB...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Concentration inhibiting [3H]-propanoyl-CCK-8 binding to cholecystokinin type A receptor of rat pancreatic membranes. | J Med Chem 35: 4249-52 (1992) BindingDB Entry DOI: 10.7270/Q2Z60N07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Bos taurus) | BDBM50003199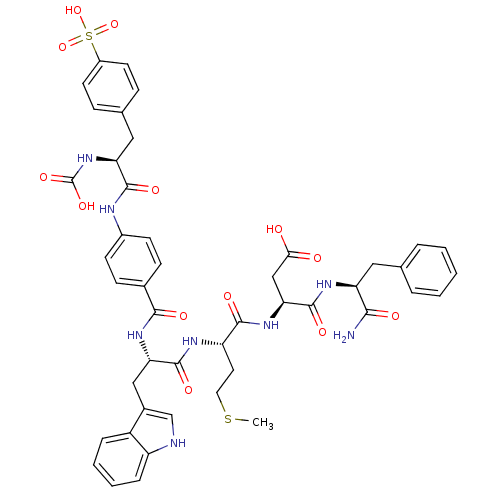 (CHEMBL126008 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0850 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of [3H]-propanoyl-CCK-8 binding to Cholecystokinin type B receptor in bovine striatum | J Med Chem 35: 3774-83 (1992) BindingDB Entry DOI: 10.7270/Q2CC0ZMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50011545 (CHEMBL430906 | Desamino-Tyr(SO3H)-Met-Gly-Trp-Met-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of [3H]-propanoyl binding to cholecystokinin type A receptor was determined in fresh rat pancreatic tissue membranes | J Med Chem 34: 1125-36 (1991) BindingDB Entry DOI: 10.7270/Q27P8XCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Bos taurus) | BDBM50003202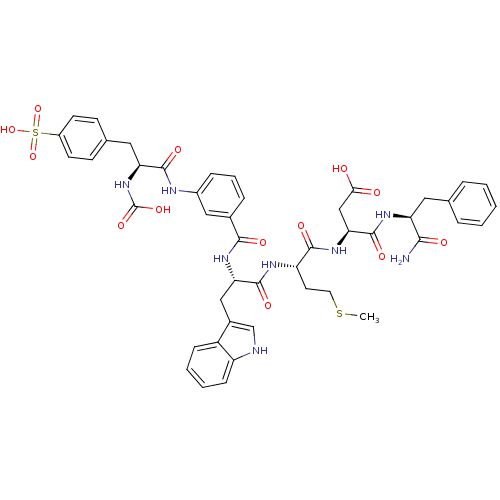 (CHEMBL331408 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of [3H]-propanoyl-CCK-8 binding to Cholecystokinin type B receptor in bovine striatum | J Med Chem 35: 3774-83 (1992) BindingDB Entry DOI: 10.7270/Q2CC0ZMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Bos taurus) | BDBM50406497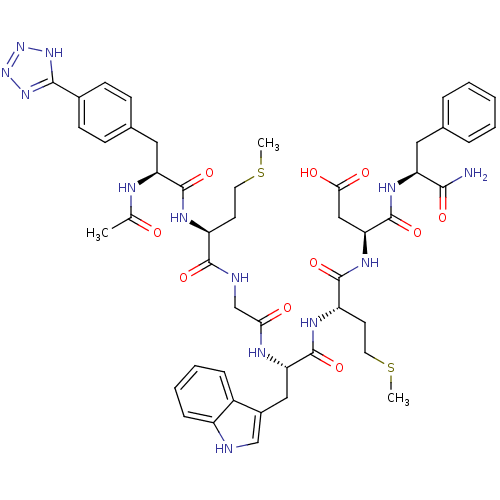 (CHEMBL2079606) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of [3H]-propanoyl binding to cholecystokinin type B receptor subtype was determined in bovine striatum membranes | J Med Chem 34: 1125-36 (1991) BindingDB Entry DOI: 10.7270/Q27P8XCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50003670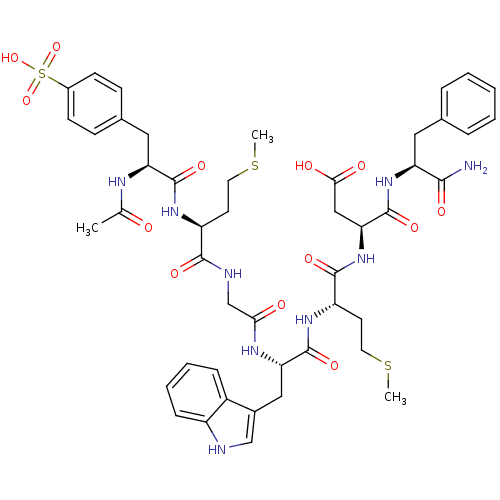 (3-{2-[2-(2-{2-[2-Acetylamino-3-(4-sulfo-phenyl)-pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Concentration inhibiting [3H]-propanoyl-CCK-8 binding to cholecystokinin type A receptor of rat pancreatic membranes. | J Med Chem 35: 4249-52 (1992) BindingDB Entry DOI: 10.7270/Q2Z60N07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50003200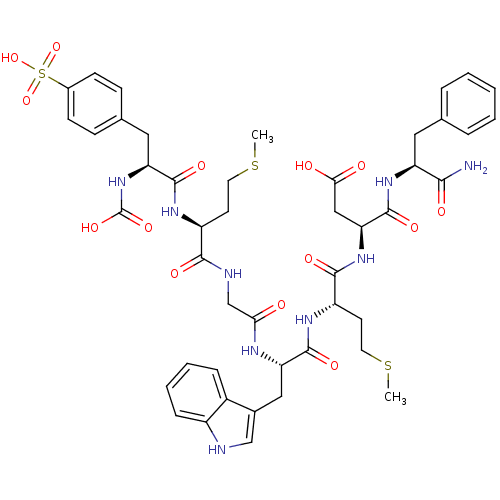 (CHEMBL267849 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description The compound was tested in vitro for inhibition of specific [3H]-propanoyl-CCK-8 binding to cholecystokinin type A receptor in rat pancreatic membran... | J Med Chem 35: 3774-83 (1992) BindingDB Entry DOI: 10.7270/Q2CC0ZMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Bos taurus) | BDBM50003201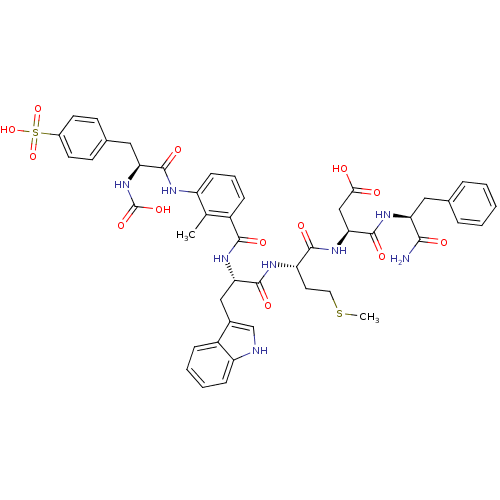 (CHEMBL386376 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of [3H]-propanoyl-CCK-8 binding to Cholecystokinin type B receptor in bovine striatum | J Med Chem 35: 3774-83 (1992) BindingDB Entry DOI: 10.7270/Q2CC0ZMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50003667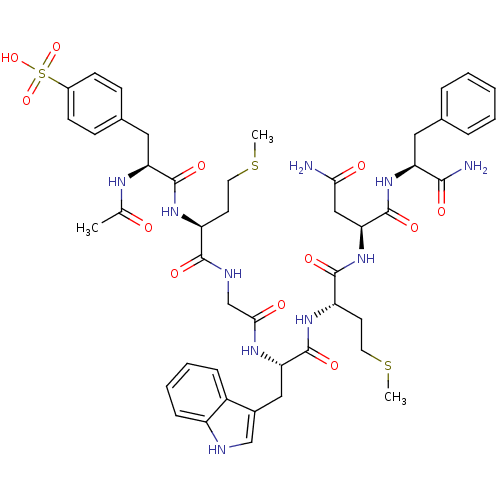 (4-{2-Acetylamino-2-[1-({[1-{1-[2-carbamoyl-1-(1-ca...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Concentration inhibiting [3H]-propanoyl-CCK-8 binding to cholecystokinin type A receptor of rat pancreatic membranes. | J Med Chem 35: 4249-52 (1992) BindingDB Entry DOI: 10.7270/Q2Z60N07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Bos taurus) | BDBM50003196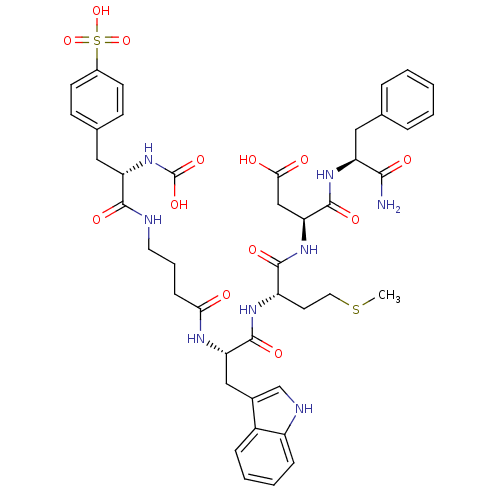 (CHEMBL433647 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of [3H]-propanoyl-CCK-8 binding to Cholecystokinin type B receptor in bovine striatum | J Med Chem 35: 3774-83 (1992) BindingDB Entry DOI: 10.7270/Q2CC0ZMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50003202 (CHEMBL331408 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description The compound was tested in vitro for inhibition of specific [3H]-propanoyl-CCK-8 binding to Cholecystokinin type A receptor in rat pancreatic membran... | J Med Chem 35: 3774-83 (1992) BindingDB Entry DOI: 10.7270/Q2CC0ZMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50003664 (4-{2-Acetylamino-2-[1-({[1-{1-[1-(1-carbamoyl-2-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Concentration inhibiting [3H]-propanoyl-CCK-8 binding to cholecystokinin type A receptor of rat pancreatic membranes. | J Med Chem 35: 4249-52 (1992) BindingDB Entry DOI: 10.7270/Q2Z60N07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50406489 (CHEMBL2079549) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of [3H]-propanoyl binding to cholecystokinin type A receptor was determined in fresh rat pancreatic tissue membranes | J Med Chem 34: 1125-36 (1991) BindingDB Entry DOI: 10.7270/Q27P8XCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50003201 (CHEMBL386376 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description The compound was tested in vitro for inhibition of specific [3H]-propanoyl-CCK-8 binding toCholecystokinin type A receptor in rat pancreatic membrane... | J Med Chem 35: 3774-83 (1992) BindingDB Entry DOI: 10.7270/Q2CC0ZMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50406497 (CHEMBL2079606) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of [3H]-propanoyl binding to cholecystokinin type A receptor was determined in fresh rat pancreatic tissue membranes | J Med Chem 34: 1125-36 (1991) BindingDB Entry DOI: 10.7270/Q27P8XCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Bos taurus) | BDBM50011545 (CHEMBL430906 | Desamino-Tyr(SO3H)-Met-Gly-Trp-Met-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of [3H]-propanoyl binding to cholecystokinin type B receptor subtype was determined in bovine striatum membranes | J Med Chem 34: 1125-36 (1991) BindingDB Entry DOI: 10.7270/Q27P8XCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Bos taurus) | BDBM50406690 (CHEMBL2112635) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of [3H]-propanoyl-CCK-8 binding to Cholecystokinin type B receptor in bovine striatum | J Med Chem 35: 3774-83 (1992) BindingDB Entry DOI: 10.7270/Q2CC0ZMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Bos taurus) | BDBM50003200 (CHEMBL267849 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of [3H]-propanoyl-CCK-8 binding to Cholecystokinin type B receptor in bovine striatum | J Med Chem 35: 3774-83 (1992) BindingDB Entry DOI: 10.7270/Q2CC0ZMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Bos taurus) | BDBM50003670 (3-{2-[2-(2-{2-[2-Acetylamino-3-(4-sulfo-phenyl)-pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Concentration of the compound inhibiting [3H]-propanoyl-CCK-8 binding to cholecystokinin type B receptor of bovine striatum. | J Med Chem 35: 4249-52 (1992) BindingDB Entry DOI: 10.7270/Q2Z60N07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50011538 (Ac-Phe(4-SO3H)-Met-Gly-Trp-Met-Asp-Phe-NH2 | CHEMB...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of [3H]-propanoyl binding to cholecystokinin type A receptor was determined in fresh rat pancreatic tissue membranes | J Med Chem 34: 1125-36 (1991) BindingDB Entry DOI: 10.7270/Q27P8XCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Bos taurus) | BDBM50011538 (Ac-Phe(4-SO3H)-Met-Gly-Trp-Met-Asp-Phe-NH2 | CHEMB...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of [3H]-propanoyl binding to cholecystokinin type B receptor subtype was determined in bovine striatum membranes | J Med Chem 34: 1125-36 (1991) BindingDB Entry DOI: 10.7270/Q27P8XCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50003196 (CHEMBL433647 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description The compound was tested in vitro for inhibition of specific [3H]-propanoyl-CCK-8 binding to cholecystokinin type A receptor in rat pancreatic membran... | J Med Chem 35: 3774-83 (1992) BindingDB Entry DOI: 10.7270/Q2CC0ZMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Bos taurus) | BDBM50011541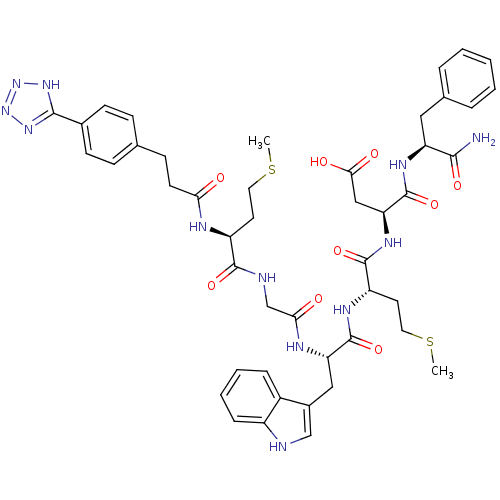 (3-4-(tetrazol-5-ylphenyl)propanoyl-Met-Gly-Trp-Met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of [3H]-propanoyl binding to cholecystokinin type B receptor subtype was determined in bovine striatum membranes | J Med Chem 34: 1125-36 (1991) BindingDB Entry DOI: 10.7270/Q27P8XCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Bos taurus) | BDBM50003207 (CHEMBL405805 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of [3H]-propanoyl-CCK-8 binding to Cholecystokinin type B receptor in bovine striatum | J Med Chem 35: 3774-83 (1992) BindingDB Entry DOI: 10.7270/Q2CC0ZMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50003665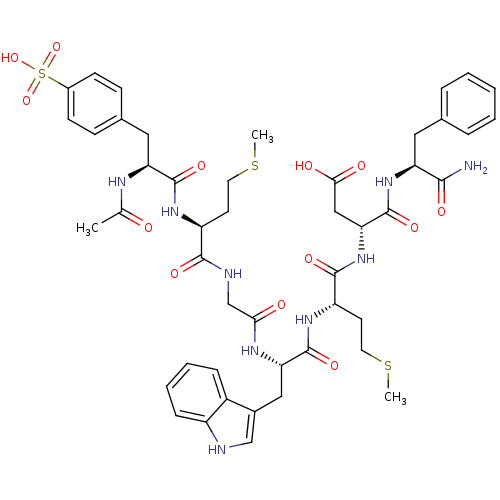 (3-{2-[2-(2-{2-[2-Acetylamino-3-(4-sulfo-phenyl)-pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Concentration inhibiting [3H]-propanoyl-CCK-8 binding to cholecystokinin type A receptor of rat pancreatic membranes. | J Med Chem 35: 4249-52 (1992) BindingDB Entry DOI: 10.7270/Q2Z60N07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50003662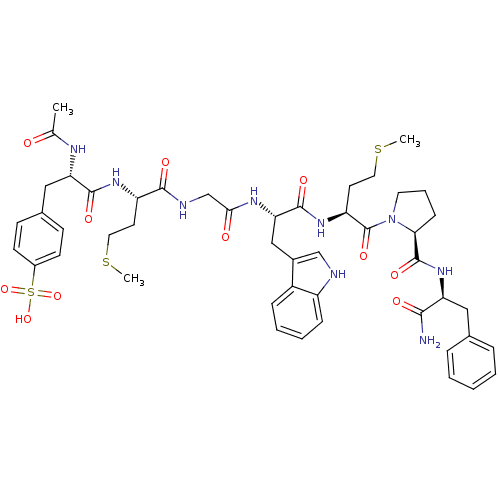 (4-{2-Acetylamino-2-[1-({[1-{1-[2-(1-carbamoyl-2-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Concentration inhibiting [3H]-propanoyl-CCK-8 binding to cholecystokinin type A receptor of rat pancreatic membranes. | J Med Chem 35: 4249-52 (1992) BindingDB Entry DOI: 10.7270/Q2Z60N07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Bos taurus) | BDBM50003205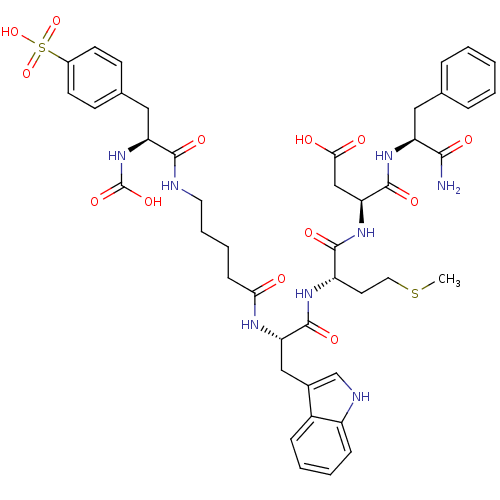 (CHEMBL421471 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of [3H]-propanoyl-CCK-8 binding to Cholecystokinin type B receptor in bovine striatum | J Med Chem 35: 3774-83 (1992) BindingDB Entry DOI: 10.7270/Q2CC0ZMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Bos taurus) | BDBM50003203 (CHEMBL436199 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of [3H]-propanoyl-CCK-8 binding to Cholecystokinin type B receptor in bovine striatum | J Med Chem 35: 3774-83 (1992) BindingDB Entry DOI: 10.7270/Q2CC0ZMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Bos taurus) | BDBM50406496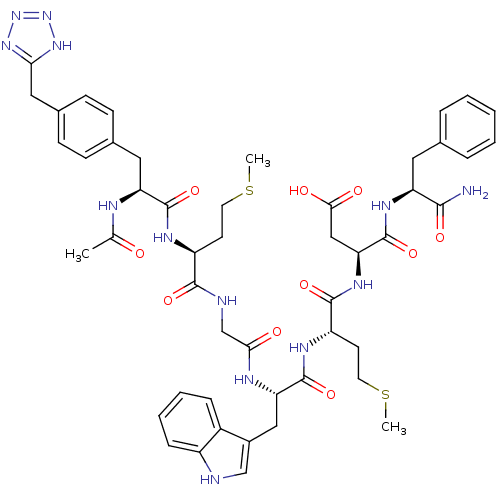 (CHEMBL2079551) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of [3H]-propanoyl binding to cholecystokinin type B receptor subtype was determined in bovine striatum membranes | J Med Chem 34: 1125-36 (1991) BindingDB Entry DOI: 10.7270/Q27P8XCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50406490 (CHEMBL2079603) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of [3H]-propanoyl binding to cholecystokinin type A receptor was determined in fresh rat pancreatic tissue membranes | J Med Chem 34: 1125-36 (1991) BindingDB Entry DOI: 10.7270/Q27P8XCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50003205 (CHEMBL421471 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description The compound was tested in vitro for inhibition of specific [3H]-propanoyl-CCK-8 binding to cholecystokinin type A receptor in rat pancreatic membran... | J Med Chem 35: 3774-83 (1992) BindingDB Entry DOI: 10.7270/Q2CC0ZMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Bos taurus) | BDBM50011542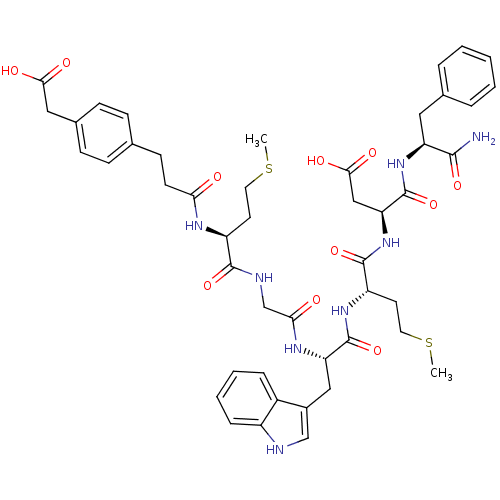 (3-[4-(carboxymethyl)phenyl]propanoyl-Met-Gly-Trp-M...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of [3H]-propanoyl binding to cholecystokinin type B receptor subtype was determined in bovine striatum membranes | J Med Chem 34: 1125-36 (1991) BindingDB Entry DOI: 10.7270/Q27P8XCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Bos taurus) | BDBM50003665 (3-{2-[2-(2-{2-[2-Acetylamino-3-(4-sulfo-phenyl)-pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Concentration inhibiting [3H]-propanoyl-CCK-8 binding to cholecystokinin type B receptor of bovine striatum. | J Med Chem 35: 4249-52 (1992) BindingDB Entry DOI: 10.7270/Q2Z60N07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Bos taurus) | BDBM50011544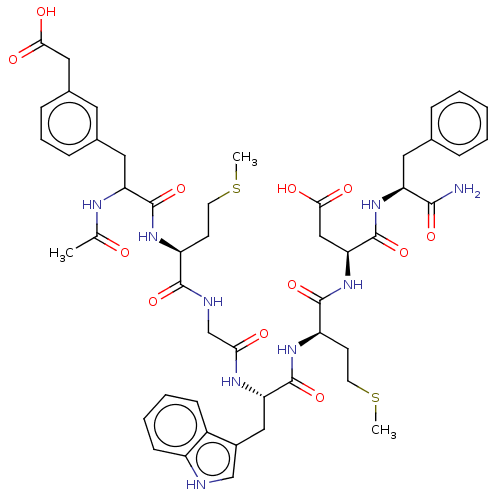 (Ac-(R,S)Phe(3-CH2COOH)-Met-Gly-Trp-Met-Asp-Phe-NH2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of [3H]-propanoyl binding to cholecystokinin type B receptor subtype was determined in bovine striatum membranes | J Med Chem 34: 1125-36 (1991) BindingDB Entry DOI: 10.7270/Q27P8XCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50011544 (Ac-(R,S)Phe(3-CH2COOH)-Met-Gly-Trp-Met-Asp-Phe-NH2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of [3H]-propanoyl binding to cholecystokinin type A receptor was determined in fresh rat pancreatic tissue membranes | J Med Chem 34: 1125-36 (1991) BindingDB Entry DOI: 10.7270/Q27P8XCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50003204 (CHEMBL122438 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description The compound was tested in vitro for inhibition of specific [3H]-propanoyl-CCK-8 binding to Cholecystokinin type A receptor in rat pancreatic membran... | J Med Chem 35: 3774-83 (1992) BindingDB Entry DOI: 10.7270/Q2CC0ZMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50011542 (3-[4-(carboxymethyl)phenyl]propanoyl-Met-Gly-Trp-M...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of [3H]-propanoyl binding to cholecystokinin type A receptor was determined in fresh rat pancreatic tissue membranes | J Med Chem 34: 1125-36 (1991) BindingDB Entry DOI: 10.7270/Q27P8XCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50406491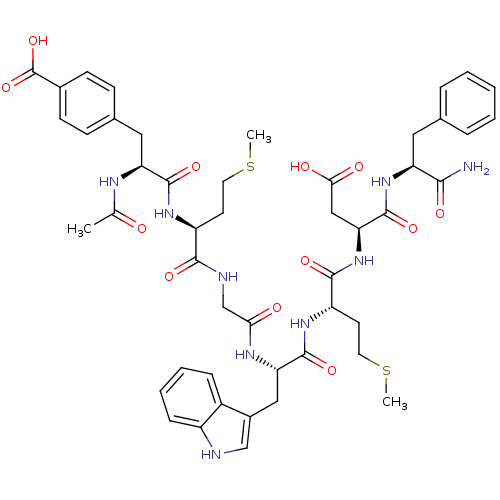 (CHEMBL2079548) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of [3H]-propanoyl binding to cholecystokinin type A receptor was determined in fresh rat pancreatic tissue membranes | J Med Chem 34: 1125-36 (1991) BindingDB Entry DOI: 10.7270/Q27P8XCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50011541 (3-4-(tetrazol-5-ylphenyl)propanoyl-Met-Gly-Trp-Met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of [3H]-propanoyl binding to cholecystokinin type A receptor was determined in fresh rat pancreatic tissue membranes | J Med Chem 34: 1125-36 (1991) BindingDB Entry DOI: 10.7270/Q27P8XCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Bos taurus) | BDBM50406489 (CHEMBL2079549) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of [3H]-propanoyl binding to cholecystokinin type B receptor subtype was determined in bovine striatum membranes | J Med Chem 34: 1125-36 (1991) BindingDB Entry DOI: 10.7270/Q27P8XCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50003668 (CHEMBL3143146 | [1-[1-[4-(1-Carbamoyl-2-phenyl-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibitory activity against cholecystokinin type A receptor from rat pancreas binding assay | J Med Chem 35: 4249-52 (1992) BindingDB Entry DOI: 10.7270/Q2Z60N07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Bos taurus) | BDBM50406491 (CHEMBL2079548) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of [3H]-propanoyl binding to cholecystokinin type B receptor subtype was determined in bovine striatum membranes | J Med Chem 34: 1125-36 (1991) BindingDB Entry DOI: 10.7270/Q27P8XCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Bos taurus) | BDBM50406492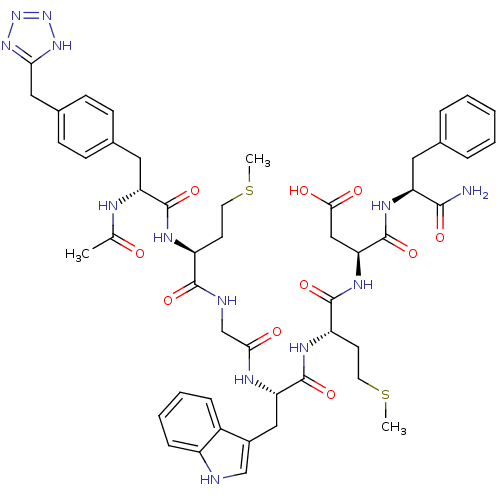 (CHEMBL2079601) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of [3H]-propanoyl binding to cholecystokinin type B receptor subtype was determined in bovine striatum membranes | J Med Chem 34: 1125-36 (1991) BindingDB Entry DOI: 10.7270/Q27P8XCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50406496 (CHEMBL2079551) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of [3H]-propanoyl binding to cholecystokinin type A receptor was determined in fresh rat pancreatic tissue membranes | J Med Chem 34: 1125-36 (1991) BindingDB Entry DOI: 10.7270/Q27P8XCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Bos taurus) | BDBM50406493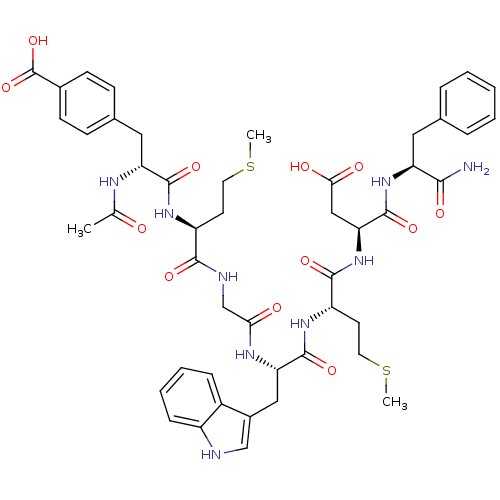 (CHEMBL2079604) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of [3H]-propanoyl binding to cholecystokinin type B receptor subtype was determined in bovine striatum membranes | J Med Chem 34: 1125-36 (1991) BindingDB Entry DOI: 10.7270/Q27P8XCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50003207 (CHEMBL405805 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description The compound was tested in vitro for inhibition of specific [3H]-propanoyl-CCK-8 binding to Cholecystokinin type A receptor in rat pancreatic membran... | J Med Chem 35: 3774-83 (1992) BindingDB Entry DOI: 10.7270/Q2CC0ZMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Bos taurus) | BDBM50406495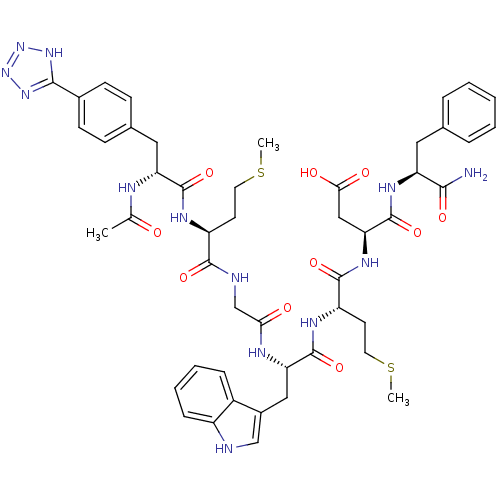 (CHEMBL2079547) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of [3H]-propanoyl binding to cholecystokinin type B receptor subtype was determined in bovine striatum membranes | J Med Chem 34: 1125-36 (1991) BindingDB Entry DOI: 10.7270/Q27P8XCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Bos taurus) | BDBM50406488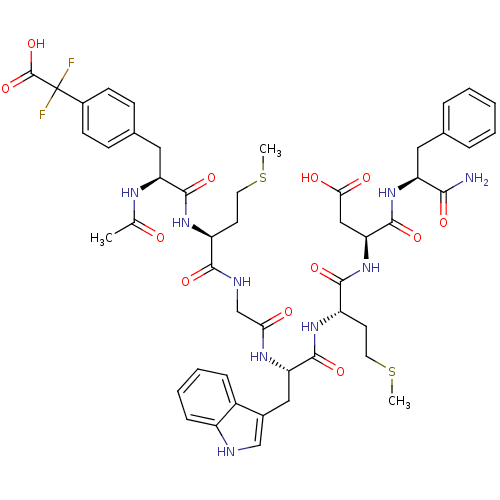 (CHEMBL2079550) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of [3H]-propanoyl binding to cholecystokinin type B receptor subtype was determined in bovine striatum membranes | J Med Chem 34: 1125-36 (1991) BindingDB Entry DOI: 10.7270/Q27P8XCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 75 total ) | Next | Last >> |