| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Acetolactate synthase, chloroplastic |
|---|
| Ligand | BDBM50487162 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_908731 (CHEMBL3066158) |
|---|
| Ki | 7750±n/a nM |
|---|
| Citation |  Chen, CN; Chen, Q; Liu, YC; Zhu, XL; Niu, CW; Xi, Z; Yang, GF Syntheses and herbicidal activity of new triazolopyrimidine-2-sulfonamides as acetohydroxyacid synthase inhibitor. Bioorg Med Chem18:4897-904 (2010) [PubMed] Article Chen, CN; Chen, Q; Liu, YC; Zhu, XL; Niu, CW; Xi, Z; Yang, GF Syntheses and herbicidal activity of new triazolopyrimidine-2-sulfonamides as acetohydroxyacid synthase inhibitor. Bioorg Med Chem18:4897-904 (2010) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Acetolactate synthase, chloroplastic |
|---|
| Name: | Acetolactate synthase, chloroplastic |
|---|
| Synonyms: | AHAS | ALS | Acetolactate synthase | CSR1 | ILVB_ARATH | TZP5 |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 72585.19 |
|---|
| Organism: | Arabidopsis thaliana |
|---|
| Description: | ChEMBL_934103 |
|---|
| Residue: | 670 |
|---|
| Sequence: | MAAATTTTTTSSSISFSTKPSPSSSKSPLPISRFSLPFSLNPNKSSSSSRRRGIKSSSPS
SISAVLNTTTNVTTTPSPTKPTKPETFISRFAPDQPRKGADILVEALERQGVETVFAYPG
GASMEIHQALTRSSSIRNVLPRHEQGGVFAAEGYARSSGKPGICIATSGPGATNLVSGLA
DALLDSVPLVAITGQVPRRMIGTDAFQETPIVEVTRSITKHNYLVMDVEDIPRIIEEAFF
LATSGRPGPVLVDVPKDIQQQLAIPNWEQAMRLPGYMSRMPKPPEDSHLEQIVRLISESK
KPVLYVGGGCLNSSDELGRFVELTGIPVASTLMGLGSYPCDDELSLHMLGMHGTVYANYA
VEHSDLLLAFGVRFDDRVTGKLEAFASRAKIVHIDIDSAEIGKNKTPHVSVCGDVKLALQ
GMNKVLENRAEELKLDFGVWRNELNVQKQKFPLSFKTFGEAIPPQYAIKVLDELTDGKAI
ISTGVGQHQMWAAQFYNYKKPRQWLSSGGLGAMGFGLPAAIGASVANPDAIVVDIDGDGS
FIMNVQELATIRVENLPVKVLLLNNQHLGMVMQWEDRFYKANRAHTFLGDPAQEDEIFPN
MLLFAAACGIPAARVTKKADLREAIQTMLDTPGPYLLDVICPHQEHVLPMIPSGGTFNDV
ITEGDGRIKY
|
|
|
|---|
| BDBM50487162 |
|---|
| n/a |
|---|
| Name | BDBM50487162 |
|---|
| Synonyms: | CHEMBL2251953 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C14H14ClN5O2S |
|---|
| Mol. Mass. | 351.811 |
|---|
| SMILES | Cc1cc2nc(nn2c(C)n1)S(=O)(=O)Nc1cc(Cl)ccc1C |
|---|
| Structure | 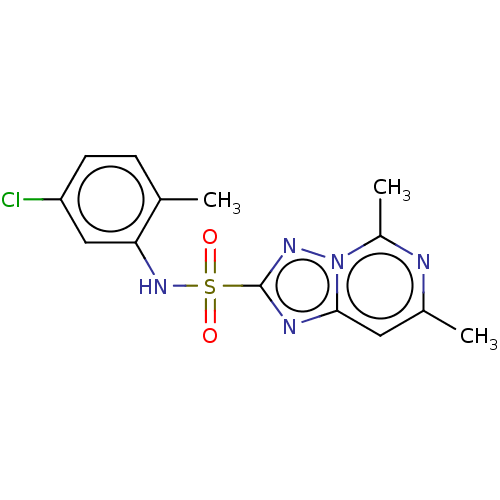
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Chen, CN; Chen, Q; Liu, YC; Zhu, XL; Niu, CW; Xi, Z; Yang, GF Syntheses and herbicidal activity of new triazolopyrimidine-2-sulfonamides as acetohydroxyacid synthase inhibitor. Bioorg Med Chem18:4897-904 (2010) [PubMed] Article
Chen, CN; Chen, Q; Liu, YC; Zhu, XL; Niu, CW; Xi, Z; Yang, GF Syntheses and herbicidal activity of new triazolopyrimidine-2-sulfonamides as acetohydroxyacid synthase inhibitor. Bioorg Med Chem18:4897-904 (2010) [PubMed] Article