 Found 703 hits with Last Name = 'yang' and Initial = 'gf'
Found 703 hits with Last Name = 'yang' and Initial = 'gf' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome b
(Sus scrofa) | BDBM50487138

(CHEMBL2251795)Show SMILES CO\C=C(\C(=O)OC)c1ccccc1COc1cc(nn1C)-c1cc(Cl)ccc1O Show InChI InChI=1S/C22H21ClN2O5/c1-25-21(11-19(24-25)17-10-15(23)8-9-20(17)26)30-12-14-6-4-5-7-16(14)18(13-28-2)22(27)29-3/h4-11,13,26H,12H2,1-3H3/b18-13+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of Sus scrofa (pig) heart cytochrome bc1 complex using DBH2 as substrate by spectrophotometric analysis |
J Am Chem Soc 132: 185-94 (2010)
Article DOI: 10.1021/ja905756c
BindingDB Entry DOI: 10.7270/Q27W6G35 |
More data for this
Ligand-Target Pair | |
Cytochrome b
(Sus scrofa) | BDBM50487139

(CHEMBL2251793)Show SMILES CO\C=C(\C(=O)OC)c1ccccc1COc1cc(nn1C)-c1ccccc1O Show InChI InChI=1S/C22H22N2O5/c1-24-21(12-19(23-24)17-10-6-7-11-20(17)25)29-13-15-8-4-5-9-16(15)18(14-27-2)22(26)28-3/h4-12,14,25H,13H2,1-3H3/b18-14+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of Sus scrofa (pig) heart cytochrome bc1 complex using DBH2 as substrate by spectrophotometric analysis |
J Am Chem Soc 132: 185-94 (2010)
Article DOI: 10.1021/ja905756c
BindingDB Entry DOI: 10.7270/Q27W6G35 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50446130

(AG-014699 | AG-14447 | RUCAPARIB CAMSYLATE | Rucap...)Show SMILES CNCc1ccc(cc1)-c1[nH]c2cc(F)cc3C(=O)NCCc1c23 Show InChI InChI=1S/C19H18FN3O/c1-21-10-11-2-4-12(5-3-11)18-14-6-7-22-19(24)15-8-13(20)9-16(23-18)17(14)15/h2-5,8-9,21,23H,6-7,10H2,1H3,(H,22,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome b
(Sus scrofa) | BDBM50487144

(CHEMBL2251794)Show SMILES CO\C=C(\C(=O)OC)c1ccccc1COc1cc(nn1C)-c1cc(C)ccc1O Show InChI InChI=1S/C23H24N2O5/c1-15-9-10-21(26)18(11-15)20-12-22(25(2)24-20)30-13-16-7-5-6-8-17(16)19(14-28-3)23(27)29-4/h5-12,14,26H,13H2,1-4H3/b19-14+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of Sus scrofa (pig) heart cytochrome bc1 complex using DBH2 as substrate by spectrophotometric analysis |
J Am Chem Soc 132: 185-94 (2010)
Article DOI: 10.1021/ja905756c
BindingDB Entry DOI: 10.7270/Q27W6G35 |
More data for this
Ligand-Target Pair | |
Cytochrome b
(Sus scrofa) | BDBM50487137

(CHEBI:78780 | DNDI1724943 | PYRACLOSTROBIN)Show SMILES CON(C(=O)OC)c1ccccc1COc1ccn(n1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C19H18ClN3O4/c1-25-19(24)23(26-2)17-6-4-3-5-14(17)13-27-18-11-12-22(21-18)16-9-7-15(20)8-10-16/h3-12H,13H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of Sus scrofa (pig) heart cytochrome bc1 complex using DBH2 as substrate by spectrophotometric analysis |
J Am Chem Soc 132: 185-94 (2010)
Article DOI: 10.1021/ja905756c
BindingDB Entry DOI: 10.7270/Q27W6G35 |
More data for this
Ligand-Target Pair | |
Cytochrome b
(Sus scrofa) | BDBM50487146

(CHEMBL2251796)Show SMILES CO\N=C(\C(=O)OC)c1ccccc1COc1cc(nn1C)-c1ccccc1O Show InChI InChI=1S/C21H21N3O5/c1-24-19(12-17(22-24)16-10-6-7-11-18(16)25)29-13-14-8-4-5-9-15(14)20(23-28-3)21(26)27-2/h4-12,25H,13H2,1-3H3/b23-20+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of Sus scrofa (pig) heart cytochrome bc1 complex using DBH2 as substrate by spectrophotometric analysis |
J Am Chem Soc 132: 185-94 (2010)
Article DOI: 10.1021/ja905756c
BindingDB Entry DOI: 10.7270/Q27W6G35 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50394447

(CHEMBL2159662)Show SMILES CC(=O)c1ccccc1NC(=O)c1ccc(OCCCCC[n+]2cccc3ccccc23)cc1 Show InChI InChI=1S/C29H28N2O3/c1-22(32)26-12-4-5-13-27(26)30-29(33)24-15-17-25(18-16-24)34-21-8-2-7-19-31-20-9-11-23-10-3-6-14-28(23)31/h3-6,9-18,20H,2,7-8,19,21H2,1H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine as substrate |
Bioorg Med Chem 20: 6739-50 (2012)
Article DOI: 10.1016/j.bmc.2012.09.016
BindingDB Entry DOI: 10.7270/Q2ZW1N1Z |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50056112
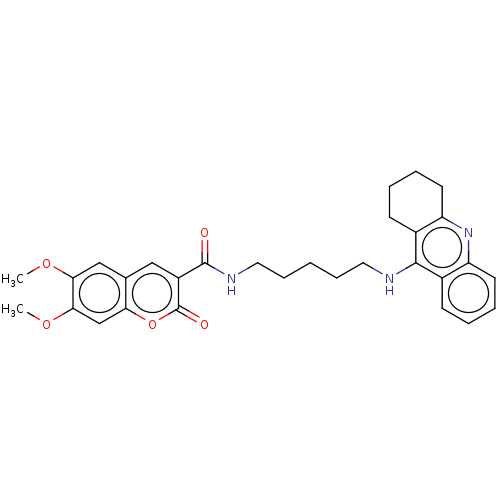
(CHEMBL3326702)Show SMILES COc1cc2cc(C(=O)NCCCCCNc3c4CCCCc4nc4ccccc34)c(=O)oc2cc1OC Show InChI InChI=1S/C30H33N3O5/c1-36-26-17-19-16-22(30(35)38-25(19)18-27(26)37-2)29(34)32-15-9-3-8-14-31-28-20-10-4-6-12-23(20)33-24-13-7-5-11-21(24)28/h4,6,10,12,16-18H,3,5,7-9,11,13-15H2,1-2H3,(H,31,33)(H,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
Coproporphyrinogen III oxidase
(Bacillus subtilis (strain 168)) | BDBM50414826
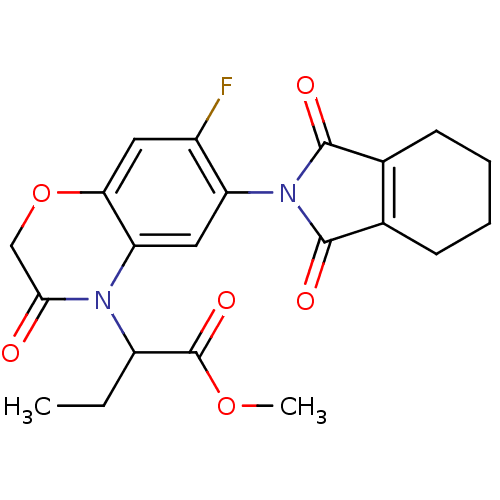
(CHEMBL575443)Show SMILES CCC(N1C(=O)COc2cc(F)c(cc12)N1C(=O)C2=C(CCCC2)C1=O)C(=O)OC |t:20| Show InChI InChI=1S/C21H21FN2O6/c1-3-14(21(28)29-2)23-16-9-15(13(22)8-17(16)30-10-18(23)25)24-19(26)11-6-4-5-7-12(11)20(24)27/h8-9,14H,3-7,10H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of Bacillus subtilis Protoporphyrinogen oxidase by capillary electrophoresis |
Bioorg Med Chem 17: 4935-42 (2009)
Article DOI: 10.1016/j.bmc.2009.06.003
BindingDB Entry DOI: 10.7270/Q2QR4ZCR |
More data for this
Ligand-Target Pair | |
Coproporphyrinogen III oxidase
(Bacillus subtilis (strain 168)) | BDBM50414833
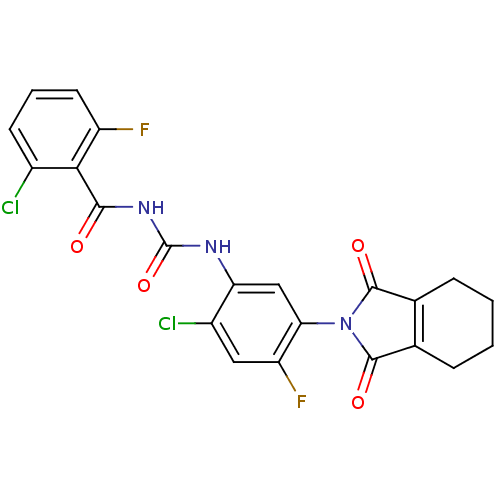
(CHEMBL573409)Show SMILES Fc1cccc(Cl)c1C(=O)NC(=O)Nc1cc(N2C(=O)C3=C(CCCC3)C2=O)c(F)cc1Cl |t:21| Show InChI InChI=1S/C22H15Cl2F2N3O4/c23-12-6-3-7-14(25)18(12)19(30)28-22(33)27-16-9-17(15(26)8-13(16)24)29-20(31)10-4-1-2-5-11(10)21(29)32/h3,6-9H,1-2,4-5H2,(H2,27,28,30,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of Bacillus subtilis Protoporphyrinogen oxidase by capillary electrophoresis |
Bioorg Med Chem 17: 4935-42 (2009)
Article DOI: 10.1016/j.bmc.2009.06.003
BindingDB Entry DOI: 10.7270/Q2QR4ZCR |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE using acetylthiocholine as substrate |
Bioorg Med Chem 20: 6739-50 (2012)
Article DOI: 10.1016/j.bmc.2012.09.016
BindingDB Entry DOI: 10.7270/Q2ZW1N1Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50394448

(CHEMBL2159661)Show SMILES CC(=O)c1ccccc1NC(=O)c1ccc(OCCCCCC[n+]2ccc3ccccc3c2)cc1 Show InChI InChI=1S/C30H30N2O3/c1-23(33)28-12-6-7-13-29(28)31-30(34)25-14-16-27(17-15-25)35-21-9-3-2-8-19-32-20-18-24-10-4-5-11-26(24)22-32/h4-7,10-18,20,22H,2-3,8-9,19,21H2,1H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine as substrate |
Bioorg Med Chem 20: 6739-50 (2012)
Article DOI: 10.1016/j.bmc.2012.09.016
BindingDB Entry DOI: 10.7270/Q2ZW1N1Z |
More data for this
Ligand-Target Pair | |
4-hydroxyphenylpyruvate dioxygenase
(Homo sapiens (Human)) | BDBM50024721
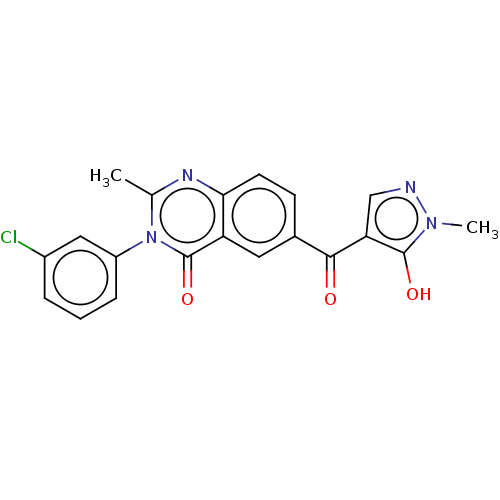
(CHEMBL3342603)Show SMILES Cc1nc2ccc(cc2c(=O)n1-c1cccc(Cl)c1)C(=O)c1cnn(C)c1O Show InChI InChI=1S/C20H15ClN4O3/c1-11-23-17-7-6-12(18(26)16-10-22-24(2)19(16)27)8-15(17)20(28)25(11)14-5-3-4-13(21)9-14/h3-10,27H,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of purified His6-tagged recombinant human HPPD assessed as inhibition of maleylacetoacetate formation after 30 mins by UV/visible spectrop... |
Bioorg Med Chem 22: 5194-211 (2014)
Article DOI: 10.1016/j.bmc.2014.08.011
BindingDB Entry DOI: 10.7270/Q2DF6SRG |
More data for this
Ligand-Target Pair | |
Succinate dehydrogenase cytochrome b560 subunit, mitochondrial
(Sus scrofa) | BDBM50079610
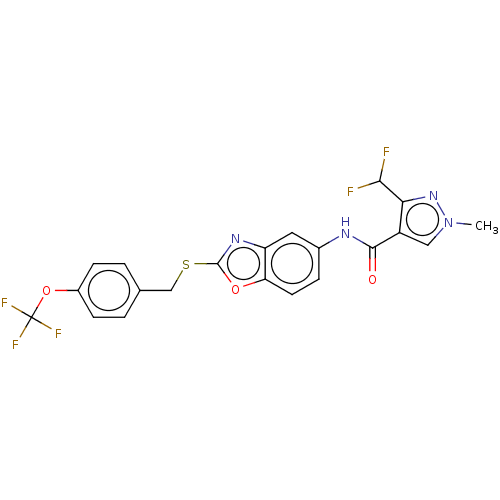
(CHEMBL3417782)Show SMILES Cn1cc(C(=O)Nc2ccc3oc(SCc4ccc(OC(F)(F)F)cc4)nc3c2)c(n1)C(F)F Show InChI InChI=1S/C21H15F5N4O3S/c1-30-9-14(17(29-30)18(22)23)19(31)27-12-4-7-16-15(8-12)28-20(32-16)34-10-11-2-5-13(6-3-11)33-21(24,25)26/h2-9,18H,10H2,1H3,(H,27,31) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of porcine heart SQR in SCR complex with respect to DCIP substrate at 23 degC by double-reciprocal plot |
Eur J Med Chem 95: 424-34 (2015)
Article DOI: 10.1016/j.ejmech.2015.03.060
BindingDB Entry DOI: 10.7270/Q27946D8 |
More data for this
Ligand-Target Pair | |
4-hydroxyphenylpyruvate dioxygenase
(Homo sapiens (Human)) | BDBM50024752
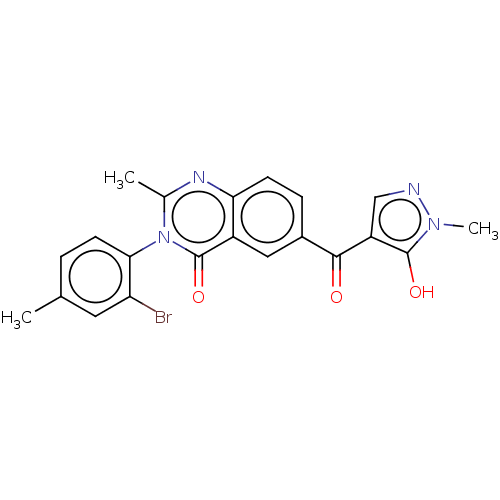
(CHEMBL3343183)Show SMILES Cc1ccc(c(Br)c1)-n1c(C)nc2ccc(cc2c1=O)C(=O)c1cnn(C)c1O |(7.71,-46.18,;6.4,-46.98,;6.43,-48.53,;5.12,-49.32,;3.77,-48.58,;3.73,-47.05,;2.38,-46.31,;5.04,-46.24,;2.46,-49.38,;2.49,-50.93,;3.84,-51.67,;1.16,-51.73,;-.2,-50.99,;-1.52,-51.78,;-2.87,-51.04,;-2.89,-49.5,;-1.58,-48.7,;-.24,-49.44,;1.09,-48.63,;1.06,-47.09,;-4.25,-48.75,;-4.28,-47.21,;-5.56,-49.55,;-5.69,-51.08,;-7.19,-51.44,;-7.98,-50.12,;-9.51,-49.99,;-6.98,-48.95,;-7.34,-47.45,)| Show InChI InChI=1S/C21H17BrN4O3/c1-11-4-7-18(16(22)8-11)26-12(2)24-17-6-5-13(9-14(17)21(26)29)19(27)15-10-23-25(3)20(15)28/h4-10,28H,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of purified His6-tagged recombinant human HPPD assessed as inhibition of maleylacetoacetate formation after 30 mins by UV/visible spectrop... |
Bioorg Med Chem 22: 5194-211 (2014)
Article DOI: 10.1016/j.bmc.2014.08.011
BindingDB Entry DOI: 10.7270/Q2DF6SRG |
More data for this
Ligand-Target Pair | |
Coproporphyrinogen III oxidase
(Bacillus subtilis (strain 168)) | BDBM50414834
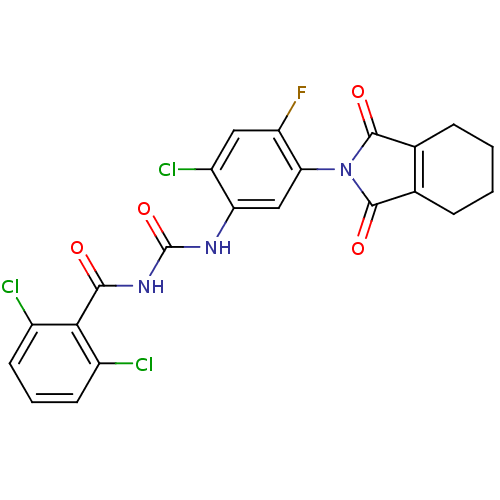
(CHEMBL573884)Show SMILES Fc1cc(Cl)c(NC(=O)NC(=O)c2c(Cl)cccc2Cl)cc1N1C(=O)C2=C(CCCC2)C1=O |t:27| Show InChI InChI=1S/C22H15Cl3FN3O4/c23-12-6-3-7-13(24)18(12)19(30)28-22(33)27-16-9-17(15(26)8-14(16)25)29-20(31)10-4-1-2-5-11(10)21(29)32/h3,6-9H,1-2,4-5H2,(H2,27,28,30,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of Bacillus subtilis Protoporphyrinogen oxidase by capillary electrophoresis |
Bioorg Med Chem 17: 4935-42 (2009)
Article DOI: 10.1016/j.bmc.2009.06.003
BindingDB Entry DOI: 10.7270/Q2QR4ZCR |
More data for this
Ligand-Target Pair | |
Cytochrome b
(Sus scrofa) | BDBM50487145
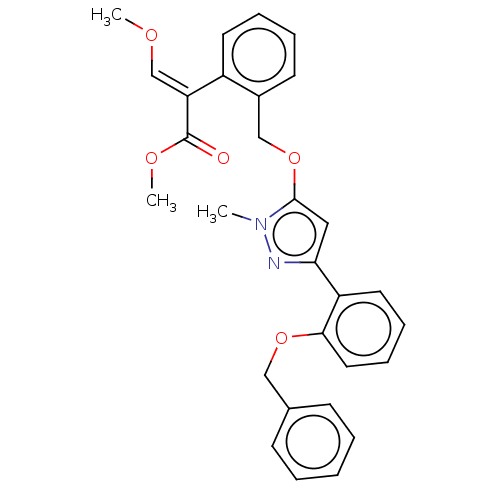
(CHEMBL2251799)Show SMILES CO\C=C(\C(=O)OC)c1ccccc1COc1cc(nn1C)-c1ccccc1OCc1ccccc1 Show InChI InChI=1S/C29H28N2O5/c1-31-28(36-19-22-13-7-8-14-23(22)25(20-33-2)29(32)34-3)17-26(30-31)24-15-9-10-16-27(24)35-18-21-11-5-4-6-12-21/h4-17,20H,18-19H2,1-3H3/b25-20+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of Sus scrofa (pig) heart cytochrome bc1 complex using DBH2 as substrate by spectrophotometric analysis |
J Am Chem Soc 132: 185-94 (2010)
Article DOI: 10.1021/ja905756c
BindingDB Entry DOI: 10.7270/Q27W6G35 |
More data for this
Ligand-Target Pair | |
4-hydroxyphenylpyruvate dioxygenase
(Homo sapiens (Human)) | BDBM50024719

(CHEMBL3342605)Show SMILES Cc1cccc(c1)-n1c(C)nc2ccc(cc2c1=O)C(=O)c1cnn(C)c1O Show InChI InChI=1S/C21H18N4O3/c1-12-5-4-6-15(9-12)25-13(2)23-18-8-7-14(10-16(18)21(25)28)19(26)17-11-22-24(3)20(17)27/h4-11,27H,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of purified His6-tagged recombinant human HPPD assessed as inhibition of maleylacetoacetate formation after 30 mins by UV/visible spectrop... |
Bioorg Med Chem 22: 5194-211 (2014)
Article DOI: 10.1016/j.bmc.2014.08.011
BindingDB Entry DOI: 10.7270/Q2DF6SRG |
More data for this
Ligand-Target Pair | |
4-hydroxyphenylpyruvate dioxygenase
(Homo sapiens (Human)) | BDBM50024720
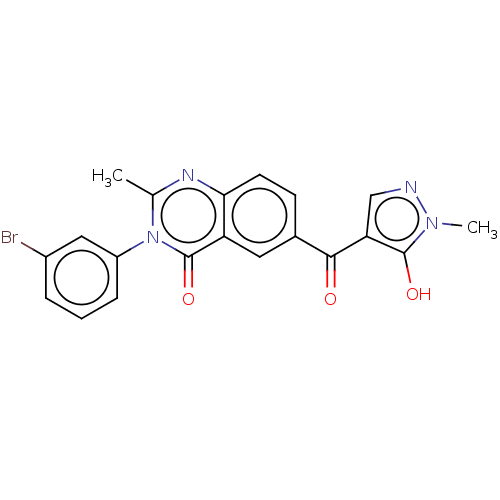
(CHEMBL3342604)Show SMILES Cc1nc2ccc(cc2c(=O)n1-c1cccc(Br)c1)C(=O)c1cnn(C)c1O Show InChI InChI=1S/C20H15BrN4O3/c1-11-23-17-7-6-12(18(26)16-10-22-24(2)19(16)27)8-15(17)20(28)25(11)14-5-3-4-13(21)9-14/h3-10,27H,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of purified His6-tagged recombinant human HPPD assessed as inhibition of maleylacetoacetate formation after 30 mins by UV/visible spectrop... |
Bioorg Med Chem 22: 5194-211 (2014)
Article DOI: 10.1016/j.bmc.2014.08.011
BindingDB Entry DOI: 10.7270/Q2DF6SRG |
More data for this
Ligand-Target Pair | |
4-hydroxyphenylpyruvate dioxygenase
(Homo sapiens (Human)) | BDBM50024727
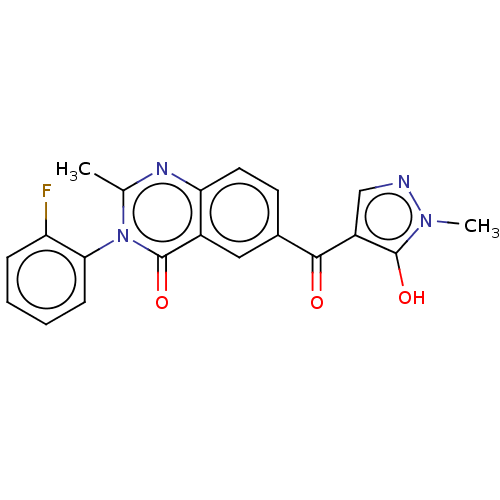
(CHEMBL3342432)Show SMILES Cc1nc2ccc(cc2c(=O)n1-c1ccccc1F)C(=O)c1cnn(C)c1O |(22.4,-6.56,;21.05,-5.81,;19.72,-6.62,;18.37,-5.87,;17.05,-6.67,;15.7,-5.92,;15.67,-4.38,;16.98,-3.59,;18.33,-4.32,;19.65,-3.51,;19.61,-1.97,;21.01,-4.26,;22.33,-3.46,;22.29,-1.93,;23.6,-1.13,;24.96,-1.87,;24.99,-3.41,;23.67,-4.21,;23.7,-5.75,;14.33,-3.64,;14.29,-2.1,;13.01,-4.43,;12.88,-5.97,;11.38,-6.32,;10.58,-5,;9.05,-4.88,;11.58,-3.84,;11.23,-2.34,)| Show InChI InChI=1S/C20H15FN4O3/c1-11-23-16-8-7-12(18(26)14-10-22-24(2)19(14)27)9-13(16)20(28)25(11)17-6-4-3-5-15(17)21/h3-10,27H,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of purified His6-tagged recombinant human HPPD assessed as inhibition of maleylacetoacetate formation after 30 mins by UV/visible spectrop... |
Bioorg Med Chem 22: 5194-211 (2014)
Article DOI: 10.1016/j.bmc.2014.08.011
BindingDB Entry DOI: 10.7270/Q2DF6SRG |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50056107
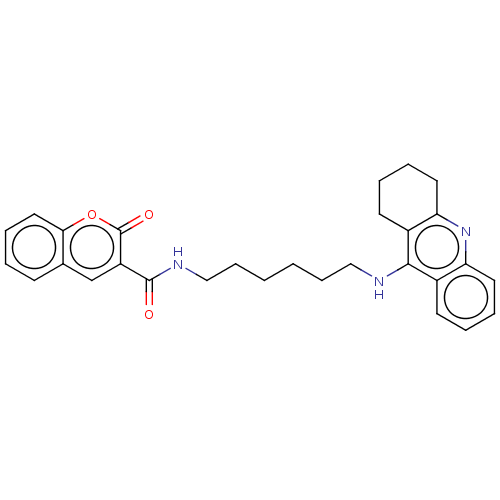
(CHEMBL3326704)Show SMILES O=C(NCCCCCCNc1c2CCCCc2nc2ccccc12)c1cc2ccccc2oc1=O Show InChI InChI=1S/C29H31N3O3/c33-28(23-19-20-11-3-8-16-26(20)35-29(23)34)31-18-10-2-1-9-17-30-27-21-12-4-6-14-24(21)32-25-15-7-5-13-22(25)27/h3-4,6,8,11-12,14,16,19H,1-2,5,7,9-10,13,15,17-18H2,(H,30,32)(H,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056107

(CHEMBL3326704)Show SMILES O=C(NCCCCCCNc1c2CCCCc2nc2ccccc12)c1cc2ccccc2oc1=O Show InChI InChI=1S/C29H31N3O3/c33-28(23-19-20-11-3-8-16-26(20)35-29(23)34)31-18-10-2-1-9-17-30-27-21-12-4-6-14-24(21)32-25-15-7-5-13-22(25)27/h3-4,6,8,11-12,14,16,19H,1-2,5,7,9-10,13,15,17-18H2,(H,30,32)(H,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50056109

(CHEMBL3326709)Show SMILES FC(F)(F)Oc1ccc2oc(=O)c(cc2c1)C(=O)NCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C30H30F3N3O4/c31-30(32,33)40-20-13-14-26-19(17-20)18-23(29(38)39-26)28(37)35-16-8-2-1-7-15-34-27-21-9-3-5-11-24(21)36-25-12-6-4-10-22(25)27/h3,5,9,11,13-14,17-18H,1-2,4,6-8,10,12,15-16H2,(H,34,36)(H,35,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
4-hydroxyphenylpyruvate dioxygenase
(Homo sapiens (Human)) | BDBM50024758

(CHEMBL3342610)Show SMILES Cc1nc2ccc(cc2c(=O)n1-c1ccc(Br)cc1)C(=O)c1cnn(C)c1O Show InChI InChI=1S/C20H15BrN4O3/c1-11-23-17-8-3-12(18(26)16-10-22-24(2)19(16)27)9-15(17)20(28)25(11)14-6-4-13(21)5-7-14/h3-10,27H,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of purified His6-tagged recombinant human HPPD assessed as inhibition of maleylacetoacetate formation after 30 mins by UV/visible spectrop... |
Bioorg Med Chem 22: 5194-211 (2014)
Article DOI: 10.1016/j.bmc.2014.08.011
BindingDB Entry DOI: 10.7270/Q2DF6SRG |
More data for this
Ligand-Target Pair | |
4-hydroxyphenylpyruvate dioxygenase
(Homo sapiens (Human)) | BDBM50024728
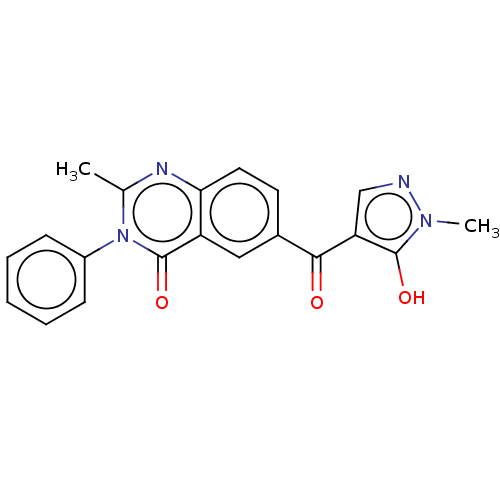
(CHEMBL3342431)Show SMILES Cc1nc2ccc(cc2c(=O)n1-c1ccccc1)C(=O)c1cnn(C)c1O Show InChI InChI=1S/C20H16N4O3/c1-12-22-17-9-8-13(18(25)16-11-21-23(2)19(16)26)10-15(17)20(27)24(12)14-6-4-3-5-7-14/h3-11,26H,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of purified His6-tagged recombinant human HPPD assessed as inhibition of maleylacetoacetate formation after 30 mins by UV/visible spectrop... |
Bioorg Med Chem 22: 5194-211 (2014)
Article DOI: 10.1016/j.bmc.2014.08.011
BindingDB Entry DOI: 10.7270/Q2DF6SRG |
More data for this
Ligand-Target Pair | |
4-hydroxyphenylpyruvate dioxygenase
(Homo sapiens (Human)) | BDBM50024722
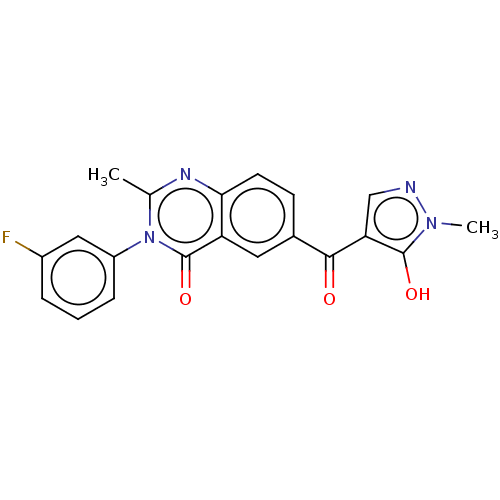
(CHEMBL3342602)Show SMILES Cc1nc2ccc(cc2c(=O)n1-c1cccc(F)c1)C(=O)c1cnn(C)c1O Show InChI InChI=1S/C20H15FN4O3/c1-11-23-17-7-6-12(18(26)16-10-22-24(2)19(16)27)8-15(17)20(28)25(11)14-5-3-4-13(21)9-14/h3-10,27H,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of purified His6-tagged recombinant human HPPD assessed as inhibition of maleylacetoacetate formation after 30 mins by UV/visible spectrop... |
Bioorg Med Chem 22: 5194-211 (2014)
Article DOI: 10.1016/j.bmc.2014.08.011
BindingDB Entry DOI: 10.7270/Q2DF6SRG |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50056119
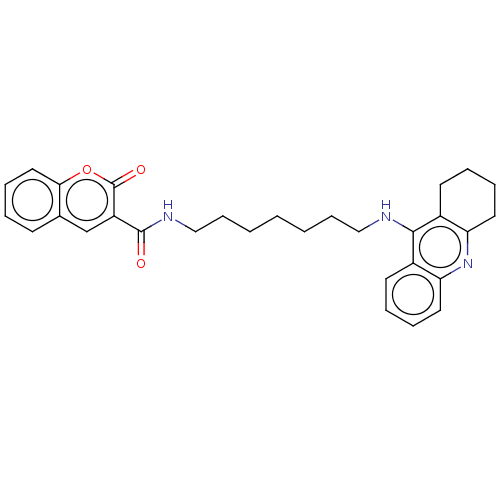
(CHEMBL3326710)Show SMILES O=C(NCCCCCCCNc1c2CCCCc2nc2ccccc12)c1cc2ccccc2oc1=O Show InChI InChI=1S/C30H33N3O3/c34-29(24-20-21-12-4-9-17-27(21)36-30(24)35)32-19-11-3-1-2-10-18-31-28-22-13-5-7-15-25(22)33-26-16-8-6-14-23(26)28/h4-5,7,9,12-13,15,17,20H,1-3,6,8,10-11,14,16,18-19H2,(H,31,33)(H,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50056122
(CHEMBL3326705)Show SMILES COc1ccc2cc(C(=O)NCCCCCCNc3c4CCCCc4nc4ccccc34)c(=O)oc2c1 Show InChI InChI=1S/C30H33N3O4/c1-36-21-15-14-20-18-24(30(35)37-27(20)19-21)29(34)32-17-9-3-2-8-16-31-28-22-10-4-6-12-25(22)33-26-13-7-5-11-23(26)28/h4,6,10,12,14-15,18-19H,2-3,5,7-9,11,13,16-17H2,1H3,(H,31,33)(H,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
Cytochrome b
(Sus scrofa) | BDBM50487142

(CHEMBL2251798)Show SMILES CO\C=C(\C(=O)OC)c1ccccc1COc1cc(nn1C)-c1cc(C)ccc1OC Show InChI InChI=1S/C24H26N2O5/c1-16-10-11-22(29-4)19(12-16)21-13-23(26(2)25-21)31-14-17-8-6-7-9-18(17)20(15-28-3)24(27)30-5/h6-13,15H,14H2,1-5H3/b20-15+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of Sus scrofa (pig) heart cytochrome bc1 complex using DBH2 as substrate by spectrophotometric analysis |
J Am Chem Soc 132: 185-94 (2010)
Article DOI: 10.1021/ja905756c
BindingDB Entry DOI: 10.7270/Q27W6G35 |
More data for this
Ligand-Target Pair | |
4-hydroxyphenylpyruvate dioxygenase
(Homo sapiens (Human)) | BDBM50024759

(CHEMBL3342609)Show SMILES Cc1nc2ccc(cc2c(=O)n1-c1ccc(Cl)cc1)C(=O)c1cnn(C)c1O Show InChI InChI=1S/C20H15ClN4O3/c1-11-23-17-8-3-12(18(26)16-10-22-24(2)19(16)27)9-15(17)20(28)25(11)14-6-4-13(21)5-7-14/h3-10,27H,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of purified His6-tagged recombinant human HPPD assessed as inhibition of maleylacetoacetate formation after 30 mins by UV/visible spectrop... |
Bioorg Med Chem 22: 5194-211 (2014)
Article DOI: 10.1016/j.bmc.2014.08.011
BindingDB Entry DOI: 10.7270/Q2DF6SRG |
More data for this
Ligand-Target Pair | |
4-hydroxyphenylpyruvate dioxygenase
(Homo sapiens (Human)) | BDBM50024726

(CHEMBL3342598)Show SMILES Cc1nc2ccc(cc2c(=O)n1-c1ccccc1Cl)C(=O)c1cnn(C)c1O |(39.95,-6.5,;38.6,-5.76,;37.27,-6.56,;35.91,-5.82,;34.6,-6.61,;33.25,-5.87,;33.22,-4.32,;34.53,-3.53,;35.88,-4.27,;37.2,-3.46,;37.16,-1.92,;38.56,-4.21,;39.88,-3.41,;39.83,-1.87,;41.14,-1.07,;42.5,-1.81,;42.54,-3.35,;41.22,-4.15,;41.25,-5.69,;31.87,-3.58,;31.84,-2.04,;30.55,-4.38,;30.42,-5.91,;28.92,-6.27,;28.13,-4.95,;26.6,-4.82,;29.13,-3.78,;28.78,-2.28,)| Show InChI InChI=1S/C20H15ClN4O3/c1-11-23-16-8-7-12(18(26)14-10-22-24(2)19(14)27)9-13(16)20(28)25(11)17-6-4-3-5-15(17)21/h3-10,27H,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of purified His6-tagged recombinant human HPPD assessed as inhibition of maleylacetoacetate formation after 30 mins by UV/visible spectrop... |
Bioorg Med Chem 22: 5194-211 (2014)
Article DOI: 10.1016/j.bmc.2014.08.011
BindingDB Entry DOI: 10.7270/Q2DF6SRG |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50056108

(CHEMBL3326707)Show SMILES Cc1ccc2oc(=O)c(cc2c1)C(=O)NCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C30H33N3O3/c1-20-14-15-27-21(18-20)19-24(30(35)36-27)29(34)32-17-9-3-2-8-16-31-28-22-10-4-6-12-25(22)33-26-13-7-5-11-23(26)28/h4,6,10,12,14-15,18-19H,2-3,5,7-9,11,13,16-17H2,1H3,(H,31,33)(H,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
4-hydroxyphenylpyruvate dioxygenase
(Homo sapiens (Human)) | BDBM50024725
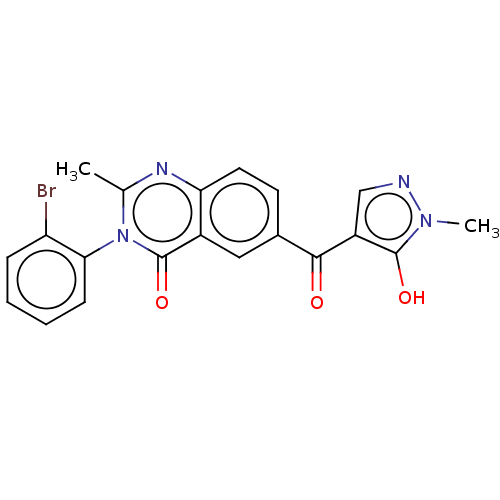
(CHEMBL3342599)Show SMILES Cc1nc2ccc(cc2c(=O)n1-c1ccccc1Br)C(=O)c1cnn(C)c1O |(57.5,-6.26,;56.15,-5.52,;54.82,-6.32,;53.47,-5.58,;52.15,-6.37,;50.8,-5.63,;50.78,-4.08,;52.09,-3.29,;53.43,-4.03,;54.75,-3.22,;54.72,-1.68,;56.12,-3.97,;57.43,-3.17,;57.39,-1.63,;58.7,-.83,;60.06,-1.57,;60.09,-3.11,;58.78,-3.91,;58.81,-5.45,;49.43,-3.34,;49.4,-1.8,;48.11,-4.14,;47.98,-5.67,;46.48,-6.03,;45.69,-4.71,;44.15,-4.58,;46.69,-3.54,;46.33,-2.04,)| Show InChI InChI=1S/C20H15BrN4O3/c1-11-23-16-8-7-12(18(26)14-10-22-24(2)19(14)27)9-13(16)20(28)25(11)17-6-4-3-5-15(17)21/h3-10,27H,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of purified His6-tagged recombinant human HPPD assessed as inhibition of maleylacetoacetate formation after 30 mins by UV/visible spectrop... |
Bioorg Med Chem 22: 5194-211 (2014)
Article DOI: 10.1016/j.bmc.2014.08.011
BindingDB Entry DOI: 10.7270/Q2DF6SRG |
More data for this
Ligand-Target Pair | |
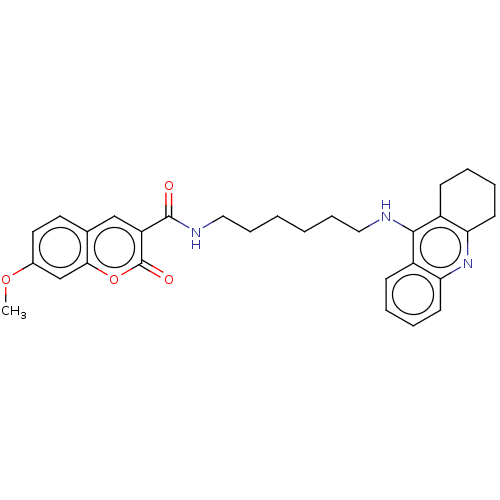
Cholinesterase
(Homo sapiens (Human)) | BDBM50056121
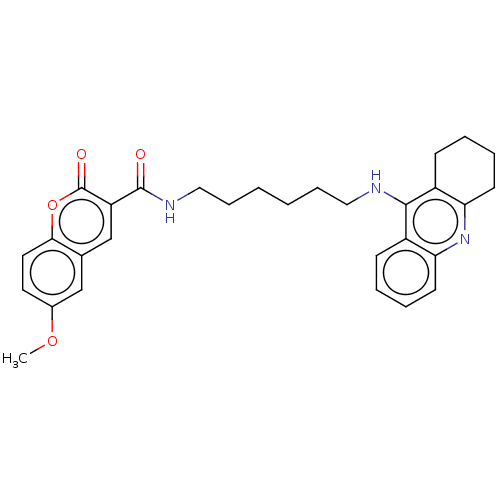
(CHEMBL3326706)Show SMILES COc1ccc2oc(=O)c(cc2c1)C(=O)NCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C30H33N3O4/c1-36-21-14-15-27-20(18-21)19-24(30(35)37-27)29(34)32-17-9-3-2-8-16-31-28-22-10-4-6-12-25(22)33-26-13-7-5-11-23(26)28/h4,6,10,12,14-15,18-19H,2-3,5,7-9,11,13,16-17H2,1H3,(H,31,33)(H,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056121

(CHEMBL3326706)Show SMILES COc1ccc2oc(=O)c(cc2c1)C(=O)NCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C30H33N3O4/c1-36-21-14-15-27-20(18-21)19-24(30(35)37-27)29(34)32-17-9-3-2-8-16-31-28-22-10-4-6-12-25(22)33-26-13-7-5-11-23(26)28/h4,6,10,12,14-15,18-19H,2-3,5,7-9,11,13,16-17H2,1H3,(H,31,33)(H,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
4-hydroxyphenylpyruvate dioxygenase
(Homo sapiens (Human)) | BDBM50024751

(CHEMBL3343184)Show SMILES Cc1nc2ccc(cc2c(=O)n1-c1ccc(Cl)c(Cl)c1)C(=O)c1cnn(C)c1O Show InChI InChI=1S/C20H14Cl2N4O3/c1-10-24-17-6-3-11(18(27)14-9-23-25(2)19(14)28)7-13(17)20(29)26(10)12-4-5-15(21)16(22)8-12/h3-9,28H,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of purified His6-tagged recombinant human HPPD assessed as inhibition of maleylacetoacetate formation after 30 mins by UV/visible spectrop... |
Bioorg Med Chem 22: 5194-211 (2014)
Article DOI: 10.1016/j.bmc.2014.08.011
BindingDB Entry DOI: 10.7270/Q2DF6SRG |
More data for this
Ligand-Target Pair | |
4-hydroxyphenylpyruvate dioxygenase
(Homo sapiens (Human)) | BDBM50024757
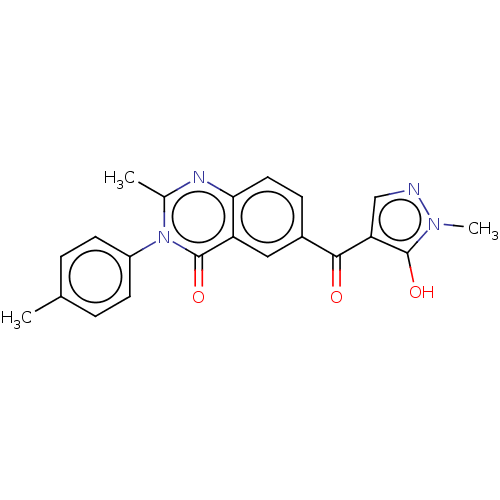
(CHEMBL3342611)Show SMILES Cc1ccc(cc1)-n1c(C)nc2ccc(cc2c1=O)C(=O)c1cnn(C)c1O Show InChI InChI=1S/C21H18N4O3/c1-12-4-7-15(8-5-12)25-13(2)23-18-9-6-14(10-16(18)21(25)28)19(26)17-11-22-24(3)20(17)27/h4-11,27H,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of purified His6-tagged recombinant human HPPD assessed as inhibition of maleylacetoacetate formation after 30 mins by UV/visible spectrop... |
Bioorg Med Chem 22: 5194-211 (2014)
Article DOI: 10.1016/j.bmc.2014.08.011
BindingDB Entry DOI: 10.7270/Q2DF6SRG |
More data for this
Ligand-Target Pair | |
Coproporphyrinogen III oxidase
(Bacillus subtilis (strain 168)) | BDBM50414827
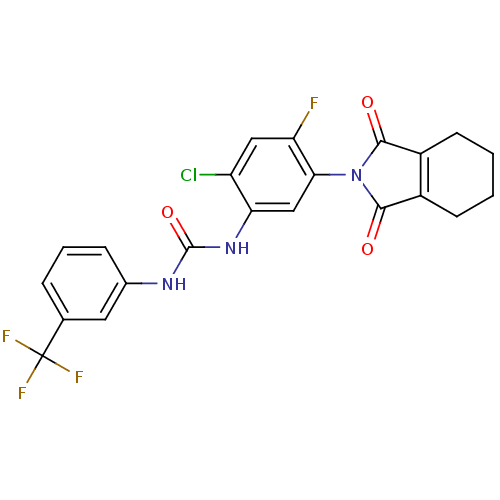
(CHEMBL576959)Show SMILES Fc1cc(Cl)c(NC(=O)Nc2cccc(c2)C(F)(F)F)cc1N1C(=O)C2=C(CCCC2)C1=O |t:27| Show InChI InChI=1S/C22H16ClF4N3O3/c23-15-9-16(24)18(30-19(31)13-6-1-2-7-14(13)20(30)32)10-17(15)29-21(33)28-12-5-3-4-11(8-12)22(25,26)27/h3-5,8-10H,1-2,6-7H2,(H2,28,29,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of Bacillus subtilis Protoporphyrinogen oxidase by capillary electrophoresis |
Bioorg Med Chem 17: 4935-42 (2009)
Article DOI: 10.1016/j.bmc.2009.06.003
BindingDB Entry DOI: 10.7270/Q2QR4ZCR |
More data for this
Ligand-Target Pair | |
Coproporphyrinogen III oxidase
(Bacillus subtilis (strain 168)) | BDBM50414837

(CHEMBL576356)Show SMILES Fc1cc(Cl)c(NC(=O)NC(=O)c2ccc(Cl)cc2Cl)cc1N1C(=O)C2=C(CCCC2)C1=O |t:27| Show InChI InChI=1S/C22H15Cl3FN3O4/c23-10-5-6-13(14(24)7-10)19(30)28-22(33)27-17-9-18(16(26)8-15(17)25)29-20(31)11-3-1-2-4-12(11)21(29)32/h5-9H,1-4H2,(H2,27,28,30,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of Bacillus subtilis Protoporphyrinogen oxidase by capillary electrophoresis |
Bioorg Med Chem 17: 4935-42 (2009)
Article DOI: 10.1016/j.bmc.2009.06.003
BindingDB Entry DOI: 10.7270/Q2QR4ZCR |
More data for this
Ligand-Target Pair | |
Coproporphyrinogen III oxidase
(Bacillus subtilis (strain 168)) | BDBM50414828
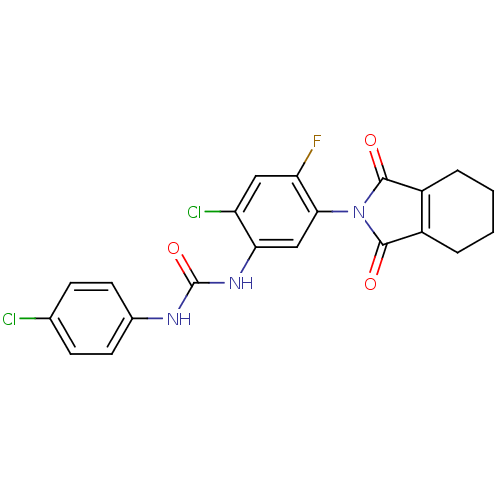
(CHEMBL573881)Show SMILES Fc1cc(Cl)c(NC(=O)Nc2ccc(Cl)cc2)cc1N1C(=O)C2=C(CCCC2)C1=O |t:24| Show InChI InChI=1S/C21H16Cl2FN3O3/c22-11-5-7-12(8-6-11)25-21(30)26-17-10-18(16(24)9-15(17)23)27-19(28)13-3-1-2-4-14(13)20(27)29/h5-10H,1-4H2,(H2,25,26,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of Bacillus subtilis Protoporphyrinogen oxidase by capillary electrophoresis |
Bioorg Med Chem 17: 4935-42 (2009)
Article DOI: 10.1016/j.bmc.2009.06.003
BindingDB Entry DOI: 10.7270/Q2QR4ZCR |
More data for this
Ligand-Target Pair | |
Cytochrome b
(Sus scrofa) | BDBM50487143

(CHEMBL2251797)Show SMILES CO\C=C(\C(=O)OC)c1ccccc1COc1cc(nn1C)-c1ccccc1OC Show InChI InChI=1S/C23H24N2O5/c1-25-22(13-20(24-25)18-11-7-8-12-21(18)28-3)30-14-16-9-5-6-10-17(16)19(15-27-2)23(26)29-4/h5-13,15H,14H2,1-4H3/b19-15+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of Sus scrofa (pig) heart cytochrome bc1 complex using DBH2 as substrate by spectrophotometric analysis |
J Am Chem Soc 132: 185-94 (2010)
Article DOI: 10.1021/ja905756c
BindingDB Entry DOI: 10.7270/Q27W6G35 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50056115
(CHEMBL3326699)Show SMILES COc1ccc2cc(C(=O)NCCCCCNc3c4CCCCc4nc4ccccc34)c(=O)oc2c1 Show InChI InChI=1S/C29H31N3O4/c1-35-20-14-13-19-17-23(29(34)36-26(19)18-20)28(33)31-16-8-2-7-15-30-27-21-9-3-5-11-24(21)32-25-12-6-4-10-22(25)27/h3,5,9,11,13-14,17-18H,2,4,6-8,10,12,15-16H2,1H3,(H,30,32)(H,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
Protoporphyrinogen oxidase, chloroplastic
(Nicotiana tabacum) | BDBM50486212
(CHEBI:9339 | SULFENTRAZONE)Show SMILES Cc1nn(-c2cc(NS(C)(=O)=O)c(Cl)cc2Cl)c(=O)n1C(F)F Show InChI InChI=1S/C11H10Cl2F2N4O3S/c1-5-16-19(11(20)18(5)10(14)15)9-4-8(17-23(2,21)22)6(12)3-7(9)13/h3-4,10,17H,1-2H3 | KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of Nicotiana tabacum (tobacco) recombinant PPO assessed as protoporphyrinogen IX formation at room temperature by fluorimetric assay |
Bioorg Med Chem 21: 3245-55 (2013)
Article DOI: 10.1016/j.bmc.2013.03.056
BindingDB Entry DOI: 10.7270/Q2ZW1PTP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056108

(CHEMBL3326707)Show SMILES Cc1ccc2oc(=O)c(cc2c1)C(=O)NCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C30H33N3O3/c1-20-14-15-27-21(18-20)19-24(30(35)36-27)29(34)32-17-9-3-2-8-16-31-28-22-10-4-6-12-25(22)33-26-13-7-5-11-23(26)28/h4,6,10,12,14-15,18-19H,2-3,5,7-9,11,13,16-17H2,1H3,(H,31,33)(H,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
4-hydroxyphenylpyruvate dioxygenase
(Homo sapiens (Human)) | BDBM50024716

(CHEMBL3342607)Show SMILES Cc1nc2ccc(cc2c(=O)n1-c1cccc(OC(F)(F)F)c1)C(=O)c1cnn(C)c1O Show InChI InChI=1S/C21H15F3N4O4/c1-11-26-17-7-6-12(18(29)16-10-25-27(2)19(16)30)8-15(17)20(31)28(11)13-4-3-5-14(9-13)32-21(22,23)24/h3-10,30H,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of purified His6-tagged recombinant human HPPD assessed as inhibition of maleylacetoacetate formation after 30 mins by UV/visible spectrop... |
Bioorg Med Chem 22: 5194-211 (2014)
Article DOI: 10.1016/j.bmc.2014.08.011
BindingDB Entry DOI: 10.7270/Q2DF6SRG |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50056120
(CHEMBL3326708)Show SMILES COc1cc2cc(C(=O)NCCCCCCNc3c4CCCCc4nc4ccccc34)c(=O)oc2cc1OC Show InChI InChI=1S/C31H35N3O5/c1-37-27-18-20-17-23(31(36)39-26(20)19-28(27)38-2)30(35)33-16-10-4-3-9-15-32-29-21-11-5-7-13-24(21)34-25-14-8-6-12-22(25)29/h5,7,11,13,17-19H,3-4,6,8-10,12,14-16H2,1-2H3,(H,32,34)(H,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056122

(CHEMBL3326705)Show SMILES COc1ccc2cc(C(=O)NCCCCCCNc3c4CCCCc4nc4ccccc34)c(=O)oc2c1 Show InChI InChI=1S/C30H33N3O4/c1-36-21-15-14-20-18-24(30(35)37-27(20)19-21)29(34)32-17-9-3-2-8-16-31-28-22-10-4-6-12-25(22)33-26-13-7-5-11-23(26)28/h4,6,10,12,14-15,18-19H,2-3,5,7-9,11,13,16-17H2,1H3,(H,31,33)(H,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE assessed as acetylthiocholine hydrolysis |
Bioorg Med Chem 22: 4784-91 (2014)
Article DOI: 10.1016/j.bmc.2014.06.057
BindingDB Entry DOI: 10.7270/Q27M09KB |
More data for this
Ligand-Target Pair | |
4-hydroxyphenylpyruvate dioxygenase
(Homo sapiens (Human)) | BDBM50024754

(CHEMBL3342614)Show SMILES Cc1cc(Cl)ccc1-n1c(C)nc2ccc(cc2c1=O)C(=O)c1cnn(C)c1O |(41.86,-39.5,;43.21,-40.23,;44.52,-39.43,;45.88,-40.17,;47.19,-39.37,;45.91,-41.71,;44.6,-42.51,;43.25,-41.77,;41.94,-42.57,;41.97,-44.12,;43.32,-44.86,;40.64,-44.92,;39.29,-44.18,;37.97,-44.97,;36.62,-44.23,;36.6,-42.69,;37.91,-41.89,;39.25,-42.63,;40.57,-41.82,;40.54,-40.28,;35.25,-41.94,;35.22,-40.4,;33.93,-42.74,;33.8,-44.27,;32.3,-44.63,;31.51,-43.31,;29.97,-43.18,;32.51,-42.14,;32.15,-40.64,)| Show InChI InChI=1S/C21H17ClN4O3/c1-11-8-14(22)5-7-18(11)26-12(2)24-17-6-4-13(9-15(17)21(26)29)19(27)16-10-23-25(3)20(16)28/h4-10,28H,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of purified His6-tagged recombinant human HPPD assessed as inhibition of maleylacetoacetate formation after 30 mins by UV/visible spectrop... |
Bioorg Med Chem 22: 5194-211 (2014)
Article DOI: 10.1016/j.bmc.2014.08.011
BindingDB Entry DOI: 10.7270/Q2DF6SRG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine as substrate |
Bioorg Med Chem 20: 6739-50 (2012)
Article DOI: 10.1016/j.bmc.2012.09.016
BindingDB Entry DOI: 10.7270/Q2ZW1N1Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data