

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50147824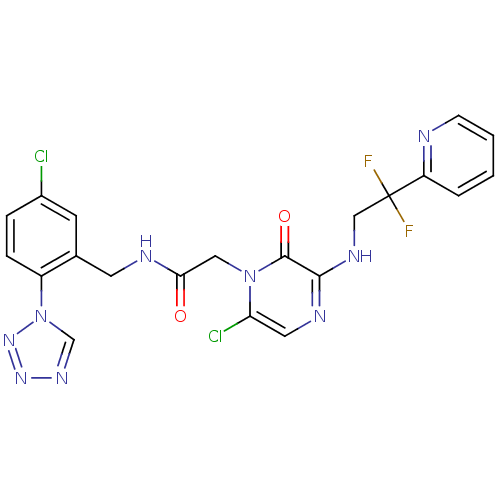 (2-[6-Chloro-3-(2,2-difluoro-2-pyridin-2-yl-ethylam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology Curated by ChEMBL | Assay Description Binding affinity to thrombin (unknown origin) | Eur J Med Chem 146: 299-317 (2018) Article DOI: 10.1016/j.ejmech.2018.01.067 BindingDB Entry DOI: 10.7270/Q2251MTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50147818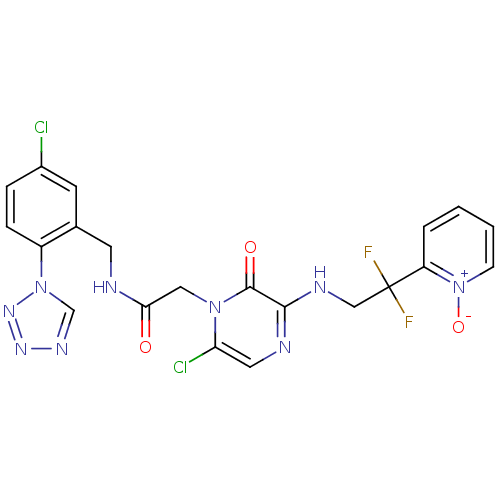 ((2-[6-CHLORO-3-{[2,2-DIFLUORO-2-(1-OXIDOPYRIDIN-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology Curated by ChEMBL | Assay Description Binding affinity to thrombin (unknown origin) | Eur J Med Chem 146: 299-317 (2018) Article DOI: 10.1016/j.ejmech.2018.01.067 BindingDB Entry DOI: 10.7270/Q2251MTJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50147818 ((2-[6-CHLORO-3-{[2,2-DIFLUORO-2-(1-OXIDOPYRIDIN-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology Curated by ChEMBL | Assay Description Inhibition of thrombin (unknown origin) | Eur J Med Chem 146: 299-317 (2018) Article DOI: 10.1016/j.ejmech.2018.01.067 BindingDB Entry DOI: 10.7270/Q2251MTJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50147824 (2-[6-Chloro-3-(2,2-difluoro-2-pyridin-2-yl-ethylam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology Curated by ChEMBL | Assay Description Inhibition of thrombin (unknown origin) | Eur J Med Chem 146: 299-317 (2018) Article DOI: 10.1016/j.ejmech.2018.01.067 BindingDB Entry DOI: 10.7270/Q2251MTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22925 ((8R)-3-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113877 BindingDB Entry DOI: 10.7270/Q2PK0M78 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM50367032 (COFORMYCIN) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113877 BindingDB Entry DOI: 10.7270/Q2PK0M78 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50457929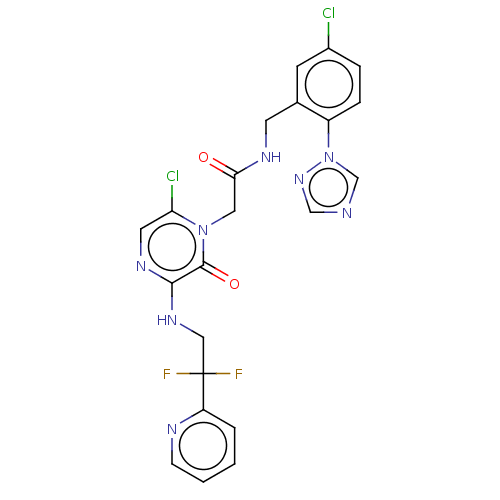 (CHEMBL104951) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology Curated by ChEMBL | Assay Description Inhibition of thrombin (unknown origin) | Eur J Med Chem 146: 299-317 (2018) Article DOI: 10.1016/j.ejmech.2018.01.067 BindingDB Entry DOI: 10.7270/Q2251MTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50457933 (CHEMBL327265) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology Curated by ChEMBL | Assay Description Inhibition of thrombin (unknown origin) | Eur J Med Chem 146: 299-317 (2018) Article DOI: 10.1016/j.ejmech.2018.01.067 BindingDB Entry DOI: 10.7270/Q2251MTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50457929 (CHEMBL104951) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology Curated by ChEMBL | Assay Description Binding affinity to thrombin (unknown origin) | Eur J Med Chem 146: 299-317 (2018) Article DOI: 10.1016/j.ejmech.2018.01.067 BindingDB Entry DOI: 10.7270/Q2251MTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50457933 (CHEMBL327265) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology Curated by ChEMBL | Assay Description Binding affinity to thrombin (unknown origin) | Eur J Med Chem 146: 299-317 (2018) Article DOI: 10.1016/j.ejmech.2018.01.067 BindingDB Entry DOI: 10.7270/Q2251MTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12751 (1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology Curated by ChEMBL | Assay Description Inhibition of human factor 10a using S-2765 as substrate after 30 mins by spectrophotometric method | Eur J Med Chem 146: 299-317 (2018) Article DOI: 10.1016/j.ejmech.2018.01.067 BindingDB Entry DOI: 10.7270/Q2251MTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50123490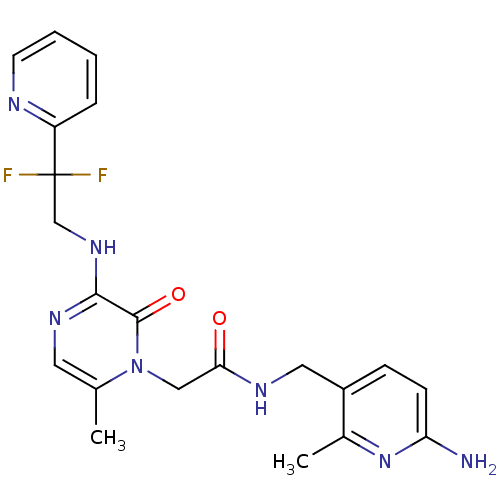 (CHEMBL143418 | N-(6-Amino-2-methyl-pyridin-3-ylmet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology Curated by ChEMBL | Assay Description Inhibition of thrombin (unknown origin) | Eur J Med Chem 146: 299-317 (2018) Article DOI: 10.1016/j.ejmech.2018.01.067 BindingDB Entry DOI: 10.7270/Q2251MTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM642537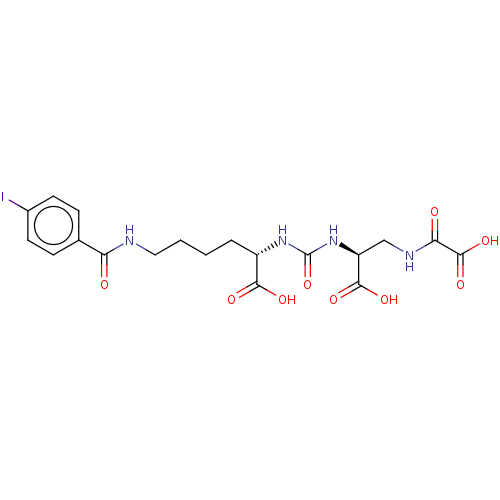 (US20230414794, Compound S2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM417026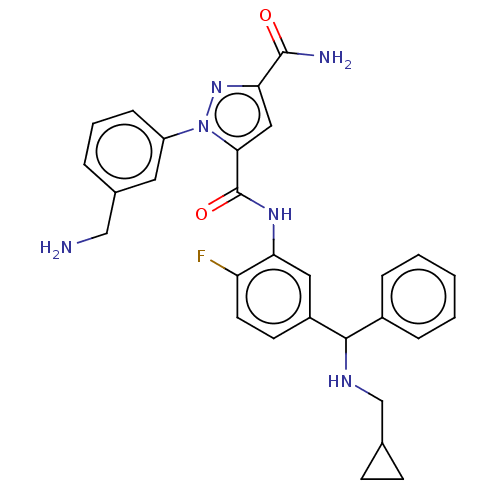 ((+-)-1-(3-(aminomethyl)phenyl)-N5-(5-((cyclopropyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM416775 (1-(3-(aminomethyl)phenyl)-N-(3-((cyclopropylmethox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50007518 ((S)-3-chloro-5-ethyl-N-((1-ethylpyrrolidin-2-yl)me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human D3R expressed in HEK293 cell membranes incubated for 1 hr by radioligand binding assay | J Med Chem 59: 10676-10691 (2016) Article DOI: 10.1021/acs.jmedchem.6b01373 BindingDB Entry DOI: 10.7270/Q2WQ05RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50104923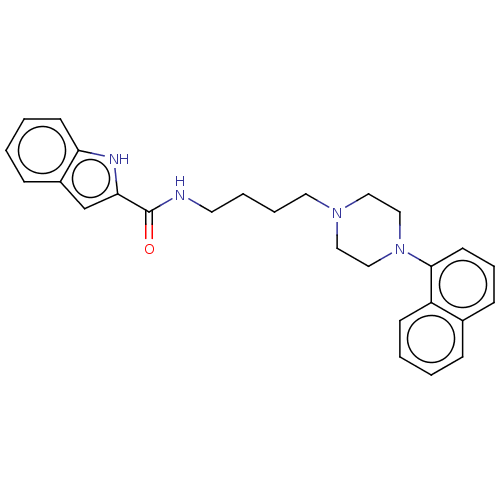 (CHEMBL3597643) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.118 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse- Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]-N-methylspiperone from human dopamine D3 receptor (unknown origin) expressed in HEK293 cells after 1 hr by liquid scintillation ... | J Med Chem 58: 6195-213 (2015) Article DOI: 10.1021/acs.jmedchem.5b00776 BindingDB Entry DOI: 10.7270/Q2KS6T92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50105009 (CHEMBL3597645) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.128 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse- Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]-N-methylspiperone from human dopamine D3 receptor (unknown origin) expressed in HEK293 cells after 1 hr by liquid scintillation ... | J Med Chem 58: 6195-213 (2015) Article DOI: 10.1021/acs.jmedchem.5b00776 BindingDB Entry DOI: 10.7270/Q2KS6T92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50533609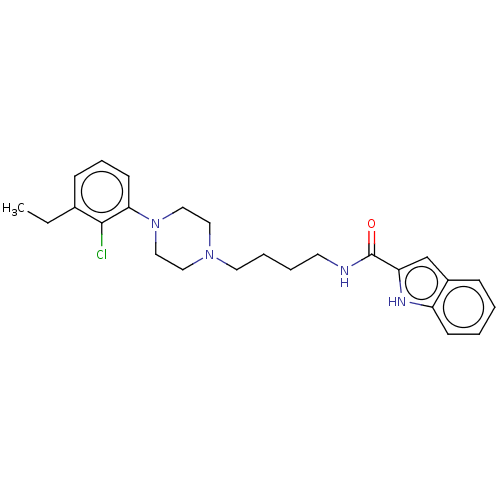 (CHEMBL4544583) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.142 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D3 receptor expressed in HEK293 cell membranes incubated for 1 hr by liquid scintillation c... | J Med Chem 59: 7634-50 (2016) Article DOI: 10.1021/acs.jmedchem.6b00860 BindingDB Entry DOI: 10.7270/Q2NS0ZDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50533620 (CHEMBL4476699) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.153 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D3 receptor expressed in HEK293 cell membranes incubated for 1 hr by liquid scintillation c... | J Med Chem 59: 7634-50 (2016) Article DOI: 10.1021/acs.jmedchem.6b00860 BindingDB Entry DOI: 10.7270/Q2NS0ZDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50104998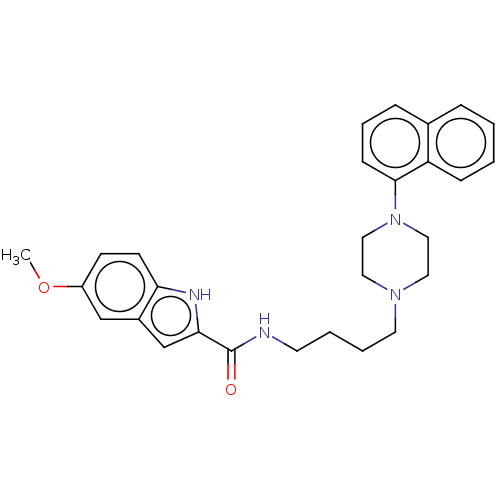 (CHEMBL3597644) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.173 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse- Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]-N-methylspiperone from human dopamine D3 receptor (unknown origin) expressed in HEK293 cells after 1 hr by liquid scintillation ... | J Med Chem 58: 6195-213 (2015) Article DOI: 10.1021/acs.jmedchem.5b00776 BindingDB Entry DOI: 10.7270/Q2KS6T92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50533625 (CHEMBL4552939) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D3 receptor expressed in HEK293 cell membranes incubated for 1 hr by liquid scintillation c... | J Med Chem 59: 7634-50 (2016) Article DOI: 10.1021/acs.jmedchem.6b00860 BindingDB Entry DOI: 10.7270/Q2NS0ZDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50193995 (3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01511 BindingDB Entry DOI: 10.7270/Q22Z19M2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50105004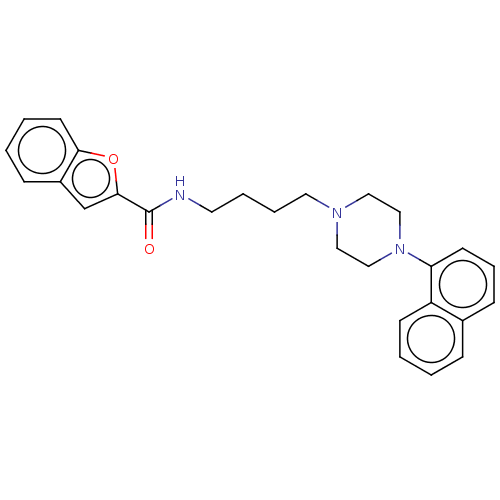 (CHEMBL3596212) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.244 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse- Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]-N-methylspiperone from human dopamine D3 receptor (unknown origin) expressed in HEK293 cells after 1 hr by liquid scintillation ... | J Med Chem 58: 6195-213 (2015) Article DOI: 10.1021/acs.jmedchem.5b00776 BindingDB Entry DOI: 10.7270/Q2KS6T92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM299269 (US9593113, Example 34) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Binding Assay (I):In order to assess the affinity of test compounds for the human glucocorticoid receptor, a commercially available kit was used (Glu... | US Patent US9593113 (2017) BindingDB Entry DOI: 10.7270/Q2833V3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50007518 ((S)-3-chloro-5-ethyl-N-((1-ethylpyrrolidin-2-yl)me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human D2R expressed in HEK293 cell membranes incubated for 1 hr by radioligand binding assay | J Med Chem 59: 10676-10691 (2016) Article DOI: 10.1021/acs.jmedchem.6b01373 BindingDB Entry DOI: 10.7270/Q2WQ05RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50601786 (CHEMBL5180162) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113877 BindingDB Entry DOI: 10.7270/Q2PK0M78 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50129425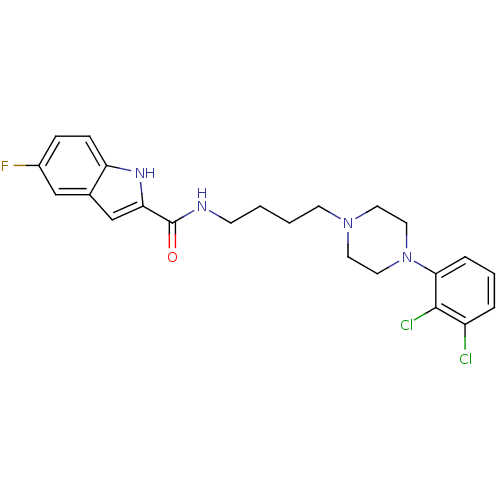 (5-Fluoro-1H-indole-2-carboxylic acid {4-[4-(2,3-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse- Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]-N-methylspiperone from human dopamine D3 receptor (unknown origin) expressed in HEK293 cells after 1 hr by liquid scintillation ... | J Med Chem 58: 6195-213 (2015) Article DOI: 10.1021/acs.jmedchem.5b00776 BindingDB Entry DOI: 10.7270/Q2KS6T92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50530563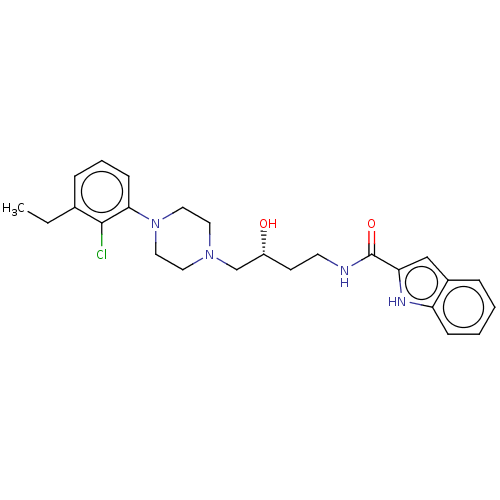 (CHEMBL4519938 | US11337971, Compound (R)-19) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human D3 receptor expressed in HEK293 cell membranes measured after 60 mins by scintillation counting meth... | J Med Chem 62: 9061-9077 (2019) Article DOI: 10.1021/acs.jmedchem.9b00607 BindingDB Entry DOI: 10.7270/Q2TF01S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50530563 (CHEMBL4519938 | US11337971, Compound (R)-19) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human D3 receptor expressed in HEK293 cell membranes measured after 60 mins by scintillation counting meth... | J Med Chem 62: 9061-9077 (2019) Article DOI: 10.1021/acs.jmedchem.9b00607 BindingDB Entry DOI: 10.7270/Q2TF01S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type/Kappa-type/Mu-type opioid receptor (MOUSE-Mus musculus (Mouse)) | BDBM50474629 (CHEMBL415006) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Compound was tested for binding affinity on intact HEK cells using [3H]diprenorphine as radioligand co-expressed with delta and kappa opioid receptor... | J Med Chem 47: 2969-72 (2004) Article DOI: 10.1021/jm0342358 BindingDB Entry DOI: 10.7270/Q2N58Q39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM299305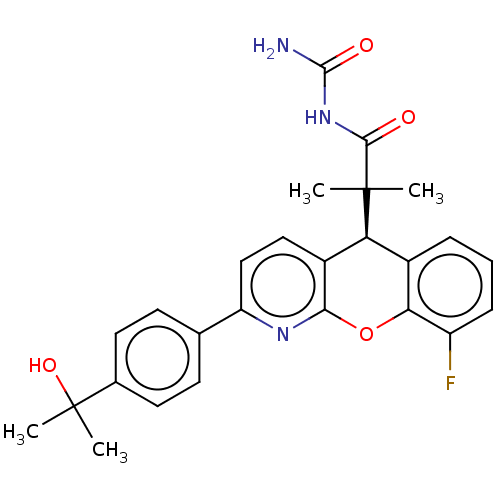 (US9593113, Example 69) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Binding Assay (I):In order to assess the affinity of test compounds for the human glucocorticoid receptor, a commercially available kit was used (Glu... | US Patent US9593113 (2017) BindingDB Entry DOI: 10.7270/Q2833V3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50104925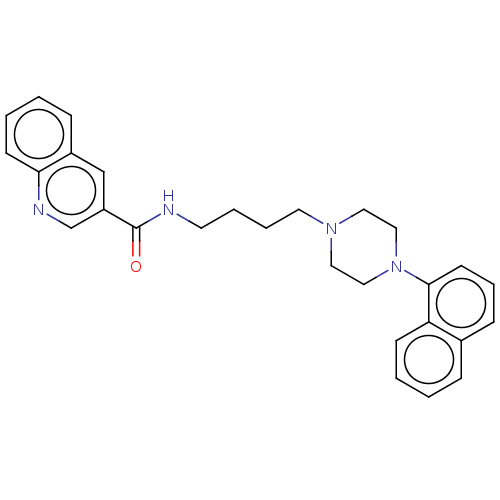 (CHEMBL3597635) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse- Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [125I]DOI from 5HT2A receptor (unknown origin) | J Med Chem 58: 6195-213 (2015) Article DOI: 10.1021/acs.jmedchem.5b00776 BindingDB Entry DOI: 10.7270/Q2KS6T92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50533617 (CHEMBL4555307) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.331 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D3 receptor expressed in HEK293 cell membranes incubated for 1 hr by liquid scintillation c... | J Med Chem 59: 7634-50 (2016) Article DOI: 10.1021/acs.jmedchem.6b00860 BindingDB Entry DOI: 10.7270/Q2NS0ZDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50533621 (CHEMBL4521947) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.341 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D3 receptor expressed in HEK293 cell membranes incubated for 1 hr by liquid scintillation c... | J Med Chem 59: 7634-50 (2016) Article DOI: 10.1021/acs.jmedchem.6b00860 BindingDB Entry DOI: 10.7270/Q2NS0ZDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50104925 (CHEMBL3597635) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.351 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse- Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]-N-methylspiperone from human dopamine D3 receptor (unknown origin) expressed in HEK293 cells after 1 hr by liquid scintillation ... | J Med Chem 58: 6195-213 (2015) Article DOI: 10.1021/acs.jmedchem.5b00776 BindingDB Entry DOI: 10.7270/Q2KS6T92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50530577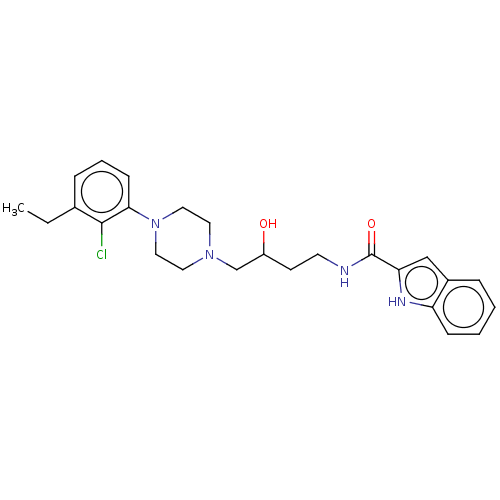 (CHEMBL4451683 | US11337971, Compound (+-)-19) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.362 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D3 receptor expressed in HEK293 cell membranes incubated for 1 hr by liquid scintillation c... | J Med Chem 59: 7634-50 (2016) Article DOI: 10.1021/acs.jmedchem.6b00860 BindingDB Entry DOI: 10.7270/Q2NS0ZDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50533612 (CHEMBL4471669) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.363 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D3 receptor expressed in HEK293 cell membranes incubated for 1 hr by liquid scintillation c... | J Med Chem 59: 7634-50 (2016) Article DOI: 10.1021/acs.jmedchem.6b00860 BindingDB Entry DOI: 10.7270/Q2NS0ZDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50530572 (CHEMBL4584497 | US11337971, Compound (R)-C5a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.373 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human D3 receptor expressed in HEK293 cell membranes measured after 60 mins by scintillation counting meth... | J Med Chem 62: 9061-9077 (2019) Article DOI: 10.1021/acs.jmedchem.9b00607 BindingDB Entry DOI: 10.7270/Q2TF01S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50530572 (CHEMBL4584497 | US11337971, Compound (R)-C5a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.373 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human D3 receptor expressed in HEK293 cell membranes measured after 60 mins by scintillation counting meth... | J Med Chem 62: 9061-9077 (2019) Article DOI: 10.1021/acs.jmedchem.9b00607 BindingDB Entry DOI: 10.7270/Q2TF01S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50104927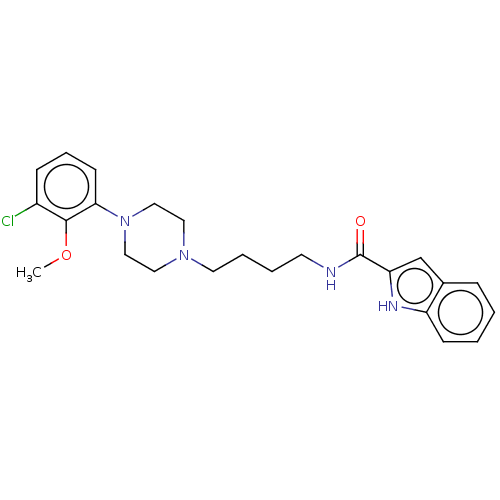 (CHEMBL3597641) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.392 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse- Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]-N-methylspiperone from human dopamine D3 receptor (unknown origin) expressed in HEK293 cells after 1 hr by liquid scintillation ... | J Med Chem 58: 6195-213 (2015) Article DOI: 10.1021/acs.jmedchem.5b00776 BindingDB Entry DOI: 10.7270/Q2KS6T92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM299270 (US9593113, Example 35 | US9593113, Example 47) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Binding Assay (I):In order to assess the affinity of test compounds for the human glucocorticoid receptor, a commercially available kit was used (Glu... | US Patent US9593113 (2017) BindingDB Entry DOI: 10.7270/Q2833V3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 4 (Homo sapiens (Human)) | BDBM50541592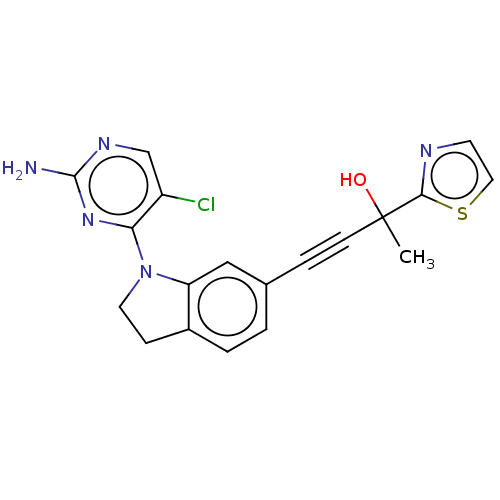 (CHEMBL3187788) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01511 BindingDB Entry DOI: 10.7270/Q22Z19M2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM299266 (US9593113, Example 31) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Binding Assay (I):In order to assess the affinity of test compounds for the human glucocorticoid receptor, a commercially available kit was used (Glu... | US Patent US9593113 (2017) BindingDB Entry DOI: 10.7270/Q2833V3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50530577 (CHEMBL4451683 | US11337971, Compound (+-)-19) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.433 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human D3 receptor expressed in HEK293 cell membranes measured after 60 mins by scintillation counting meth... | J Med Chem 62: 9061-9077 (2019) Article DOI: 10.1021/acs.jmedchem.9b00607 BindingDB Entry DOI: 10.7270/Q2TF01S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50530577 (CHEMBL4451683 | US11337971, Compound (+-)-19) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.433 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human D3 receptor expressed in HEK293 cell membranes measured after 60 mins by scintillation counting meth... | J Med Chem 62: 9061-9077 (2019) Article DOI: 10.1021/acs.jmedchem.9b00607 BindingDB Entry DOI: 10.7270/Q2TF01S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM299293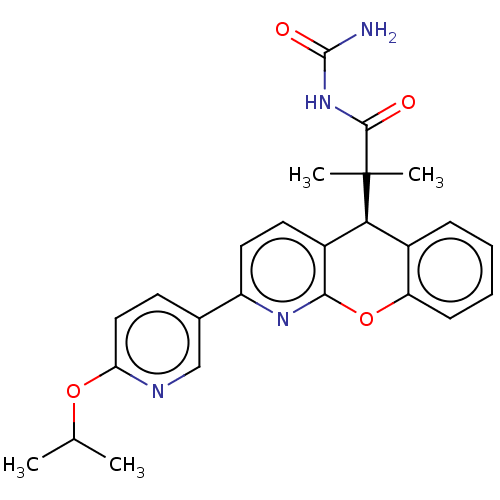 (US9593113, Example 57 | US9593113, Example 65) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Binding Assay (I):In order to assess the affinity of test compounds for the human glucocorticoid receptor, a commercially available kit was used (Glu... | US Patent US9593113 (2017) BindingDB Entry DOI: 10.7270/Q2833V3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50067796 (CHEMBL11157 | L-374087 | N-((6-amino-2-methylpyrid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology Curated by ChEMBL | Assay Description Inhibition of human thrombin assessed as release of p-nitroanilide from chromogenic substrate | Eur J Med Chem 146: 299-317 (2018) Article DOI: 10.1016/j.ejmech.2018.01.067 BindingDB Entry DOI: 10.7270/Q2251MTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type/Kappa-type/Mu-type opioid receptor (MOUSE-Mus musculus (Mouse)) | BDBM50474628 (CHEMBL386810) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Compound was tested for binding affinity on intact HEK cells using [3H]diprenorphine as radioligand co-expressed with delta and kappa opioid receptor... | J Med Chem 47: 2969-72 (2004) Article DOI: 10.1021/jm0342358 BindingDB Entry DOI: 10.7270/Q2N58Q39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (RAT) | BDBM50403547 (ATROPEN | ATROPINE) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DVanderbilt Program in Drug Discovery Curated by ChEMBL | Assay Description Displacement of [3H]NMS from rat recombinant muscarinic M4 receptor expressed in CHO cells after 2 hrs by microplate scintillation counting | Nat Chem Biol 4: 42-50 (2007) Article DOI: 10.1038/nchembio.2007.55 BindingDB Entry DOI: 10.7270/Q2D50N55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 15217 total ) | Next | Last >> |