| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Gag-Pol polyprotein [489-587] |
|---|
| Ligand | BDBM50062941 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_159631 (CHEMBL881815) |
|---|
| IC50 | 1.9±n/a nM |
|---|
| Citation |  Kempf, DJ; Sham, HL; Marsh, KC; Flentge, CA; Betebenner, D; Green, BE; McDonald, E; Vasavanonda, S; Saldivar, A; Wideburg, NE; Kati, WM; Ruiz, L; Zhao, C; Fino, L; Patterson, J; Molla, A; Plattner, JJ; Norbeck, DW Discovery of ritonavir, a potent inhibitor of HIV protease with high oral bioavailability and clinical efficacy. J Med Chem41:602-17 (1998) [PubMed] Article Kempf, DJ; Sham, HL; Marsh, KC; Flentge, CA; Betebenner, D; Green, BE; McDonald, E; Vasavanonda, S; Saldivar, A; Wideburg, NE; Kati, WM; Ruiz, L; Zhao, C; Fino, L; Patterson, J; Molla, A; Plattner, JJ; Norbeck, DW Discovery of ritonavir, a potent inhibitor of HIV protease with high oral bioavailability and clinical efficacy. J Med Chem41:602-17 (1998) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Gag-Pol polyprotein [489-587] |
|---|
| Name: | Gag-Pol polyprotein [489-587] |
|---|
| Synonyms: | Human immunodeficiency virus type 1 protease | POL_HV1H2 | Pol polyprotein | gag-pol |
|---|
| Type: | Enzyme Subunit |
|---|
| Mol. Mass.: | 10781.16 |
|---|
| Organism: | Human immunodeficiency virus type 1 |
|---|
| Description: | P04585[489-587] |
|---|
| Residue: | 99 |
|---|
| Sequence: | PQVTLWQRPLVTIKIGGQLKEALLDTGADDTVLEEMSLPGRWKPKMIGGIGGFIKVRQYD
QILIEICGHKAIGTVLVGPTPVNIIGRNLLTQIGCTLNF
|
|
|
|---|
| BDBM50062941 |
|---|
| n/a |
|---|
| Name | BDBM50062941 |
|---|
| Synonyms: | ((1S,3S,4S)-1-Benzyl-4-{2-[3-cyclopropyl-3-(2-isopropyl-thiazol-4-ylmethyl)-ureido]-3-methyl-butyrylamino}-3-hydroxy-5-phenyl-pentyl)-carbamic acid thiazol-5-ylmethyl ester | CHEMBL348147 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C39H50N6O5S2 |
|---|
| Mol. Mass. | 746.982 |
|---|
| SMILES | CC(C)C(NC(=O)N(Cc1csc(n1)C(C)C)C1CC1)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@H](Cc1ccccc1)NC(=O)OCc1cncs1 |
|---|
| Structure | 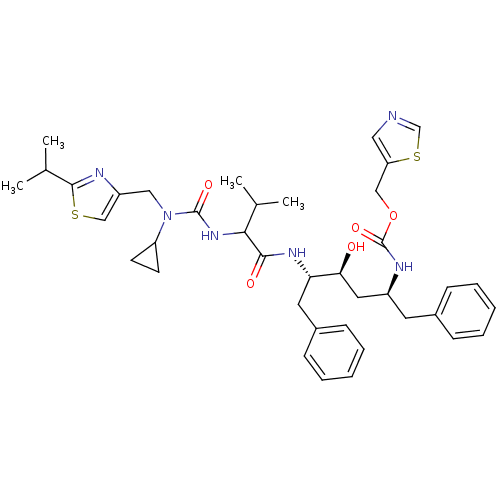
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Kempf, DJ; Sham, HL; Marsh, KC; Flentge, CA; Betebenner, D; Green, BE; McDonald, E; Vasavanonda, S; Saldivar, A; Wideburg, NE; Kati, WM; Ruiz, L; Zhao, C; Fino, L; Patterson, J; Molla, A; Plattner, JJ; Norbeck, DW Discovery of ritonavir, a potent inhibitor of HIV protease with high oral bioavailability and clinical efficacy. J Med Chem41:602-17 (1998) [PubMed] Article
Kempf, DJ; Sham, HL; Marsh, KC; Flentge, CA; Betebenner, D; Green, BE; McDonald, E; Vasavanonda, S; Saldivar, A; Wideburg, NE; Kati, WM; Ruiz, L; Zhao, C; Fino, L; Patterson, J; Molla, A; Plattner, JJ; Norbeck, DW Discovery of ritonavir, a potent inhibitor of HIV protease with high oral bioavailability and clinical efficacy. J Med Chem41:602-17 (1998) [PubMed] Article