

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM578 ((2S)-N-[(2S,4S,5S)-5-[2-(2,6-dimethylphenoxy)aceta...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 11: 1351-3 (2001) BindingDB Entry DOI: 10.7270/Q2HX1BX6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50099843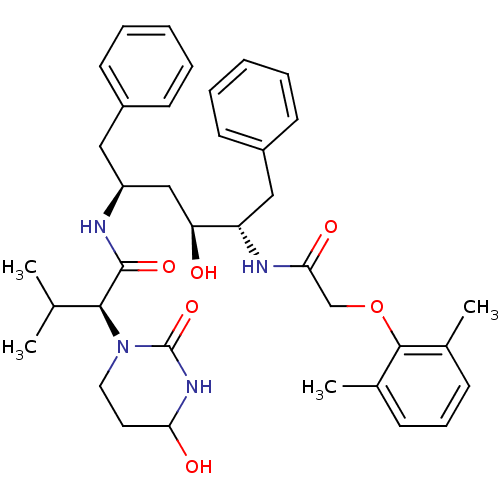 ((S)-N-[(S)-4-[2-(2,6-Dimethyl-phenoxy)-acetylamino...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 11: 1351-3 (2001) BindingDB Entry DOI: 10.7270/Q2HX1BX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50099842 ((S)-N-[(S)-4-[2-(2,6-Dimethyl-phenoxy)-acetylamino...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 11: 1351-3 (2001) BindingDB Entry DOI: 10.7270/Q2HX1BX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM194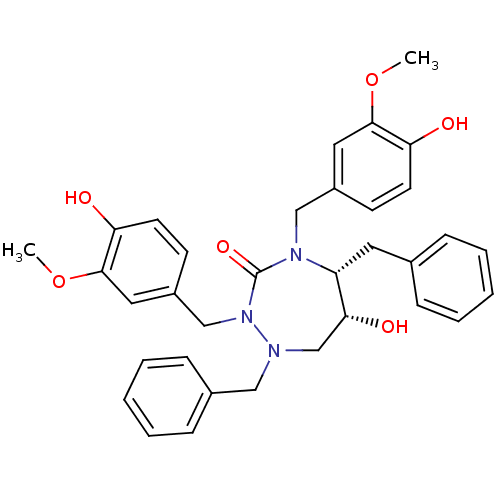 ((5R,6R)-1,5-dibenzyl-6-hydroxy-2,4-bis[(4-hydroxy-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00500 | -65.6 | n/a | n/a | n/a | n/a | n/a | 4.7 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P... | J Med Chem 39: 392-7 (1996) Article DOI: 10.1021/jm9507183 BindingDB Entry DOI: 10.7270/Q2V40SC6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50150028 ((5R,6R)-1-Benzoyl-5-benzyl-6-hydroxy-2,4-bis-(4-hy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested for inhibition of HIV protease | Bioorg Med Chem Lett 14: 4075-8 (2004) Article DOI: 10.1016/j.bmcl.2004.05.036 BindingDB Entry DOI: 10.7270/Q25B01ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM520 (1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition constant of ritonavir towards HIV protease was determined | Bioorg Med Chem Lett 7: 699-704 (1997) Article DOI: 10.1016/S0960-894X(97)00080-2 BindingDB Entry DOI: 10.7270/Q23N23C1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM520 (1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against purified HIV protease | Bioorg Med Chem Lett 5: 2725-2728 (1995) Article DOI: 10.1016/0960-894X(95)00462-3 BindingDB Entry DOI: 10.7270/Q26973JN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM195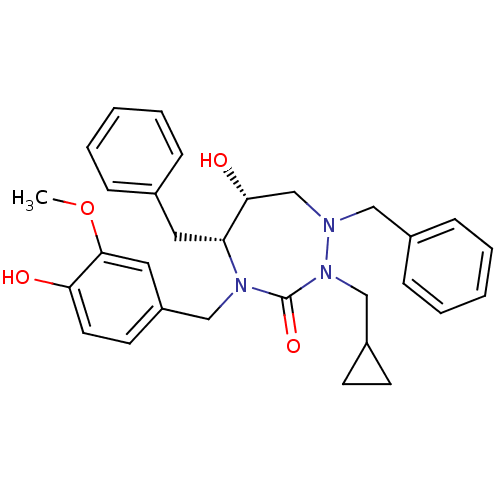 ((5R,6R)-1,5-dibenzyl-2-(cyclopropylmethyl)-6-hydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0620 | -59.2 | n/a | n/a | n/a | n/a | n/a | 4.7 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P... | J Med Chem 39: 392-7 (1996) Article DOI: 10.1021/jm9507183 BindingDB Entry DOI: 10.7270/Q2V40SC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM192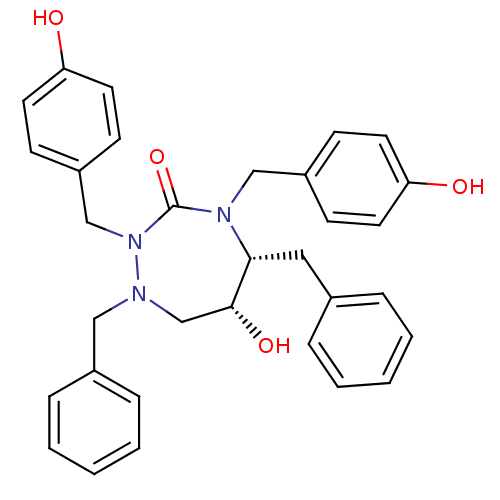 ((5R,6R)-1,5-dibenzyl-6-hydroxy-2,4-bis[(4-hydroxyp...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested for inhibition of HIV protease | Bioorg Med Chem Lett 14: 4075-8 (2004) Article DOI: 10.1016/j.bmcl.2004.05.036 BindingDB Entry DOI: 10.7270/Q25B01ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM192 ((5R,6R)-1,5-dibenzyl-6-hydroxy-2,4-bis[(4-hydroxyp...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | -58.9 | n/a | n/a | n/a | n/a | n/a | 4.7 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P... | J Med Chem 39: 392-7 (1996) Article DOI: 10.1021/jm9507183 BindingDB Entry DOI: 10.7270/Q2V40SC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM193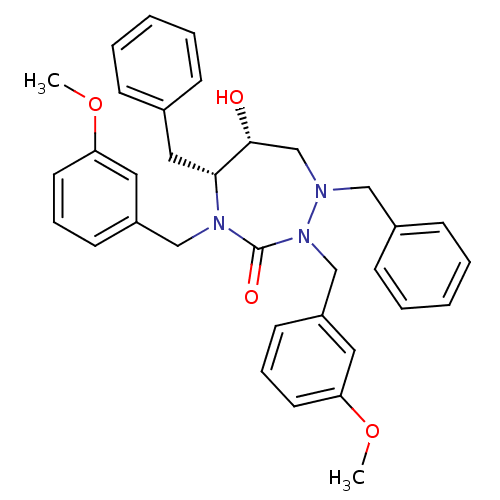 ((5R,6R)-1,5-dibenzyl-6-hydroxy-2,4-bis[(3-methoxyp...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.220 | -56.0 | n/a | n/a | n/a | n/a | n/a | 4.7 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P... | J Med Chem 39: 392-7 (1996) Article DOI: 10.1021/jm9507183 BindingDB Entry DOI: 10.7270/Q2V40SC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM189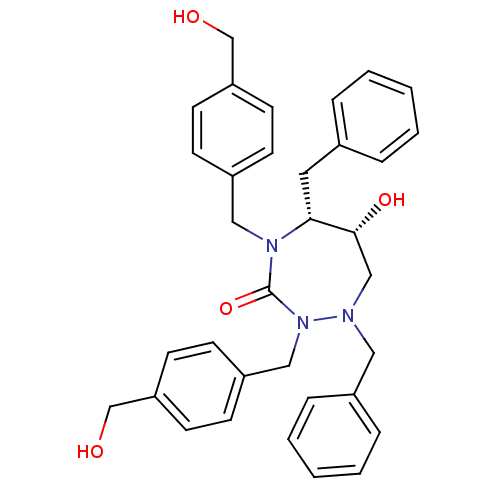 ((5R,6R)-1,5-dibenzyl-6-hydroxy-2,4-bis({[4-(hydrox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.220 | -56.0 | n/a | n/a | n/a | n/a | n/a | 4.7 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P... | J Med Chem 39: 392-7 (1996) Article DOI: 10.1021/jm9507183 BindingDB Entry DOI: 10.7270/Q2V40SC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM190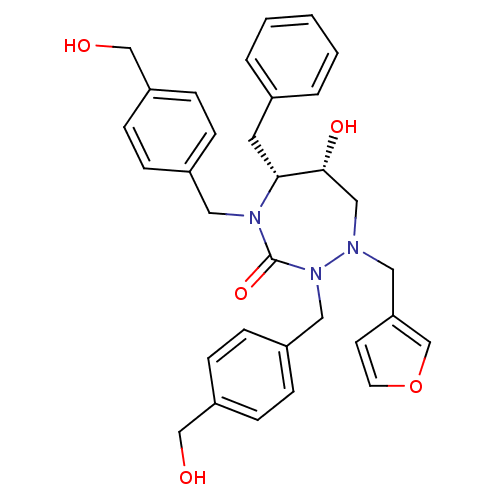 ((5R,6R)-2,4-Bis[4-(hydroxymethyl)benzyl]-1-(3-fura...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.490 | -54.0 | n/a | n/a | n/a | n/a | n/a | 4.7 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P... | J Med Chem 39: 392-7 (1996) Article DOI: 10.1021/jm9507183 BindingDB Entry DOI: 10.7270/Q2V40SC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50150033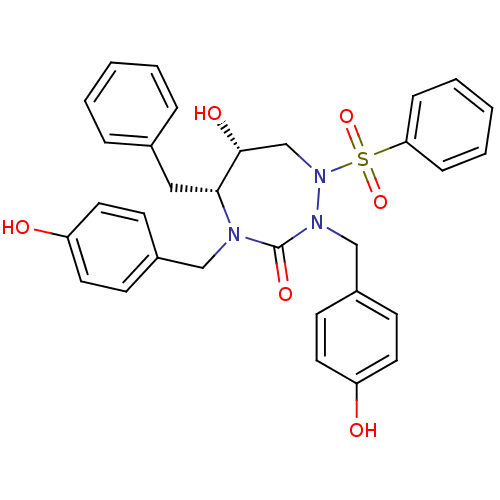 ((5R,6R)-1-Benzenesulfonyl-5-benzyl-6-hydroxy-2,4-b...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested for inhibition of HIV protease | Bioorg Med Chem Lett 14: 4075-8 (2004) Article DOI: 10.1016/j.bmcl.2004.05.036 BindingDB Entry DOI: 10.7270/Q25B01ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM191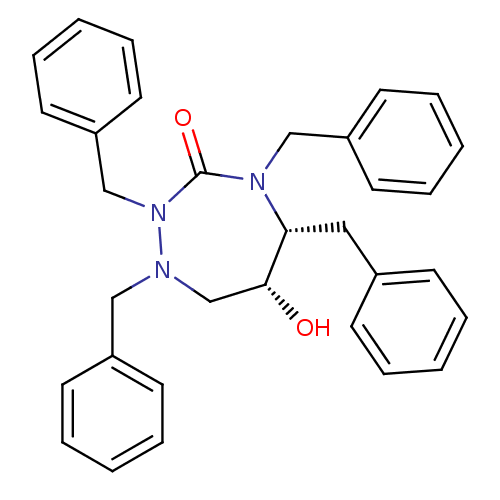 ((5R,6R)-1,2,4,5-tetrabenzyl-6-hydroxy-1,2,4-triaze...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | -50.5 | n/a | n/a | n/a | n/a | n/a | 4.7 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P... | J Med Chem 39: 392-7 (1996) Article DOI: 10.1021/jm9507183 BindingDB Entry DOI: 10.7270/Q2V40SC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50014144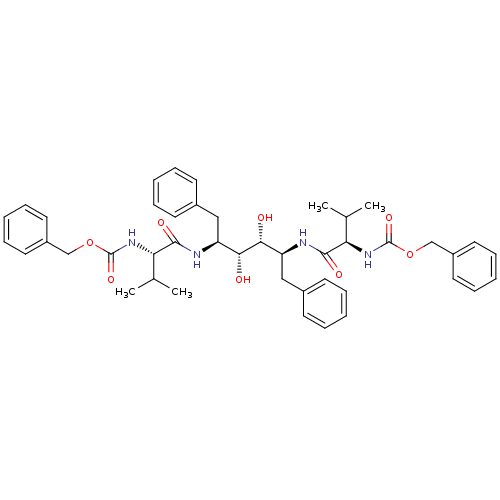 (CHEMBL83462 | {(S)-1-[(1S,2R,3R,4S)-1-Benzyl-4-((R...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against recombinant HIV-1 Protease | J Med Chem 33: 2687-9 (1990) BindingDB Entry DOI: 10.7270/Q2W37V89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50014145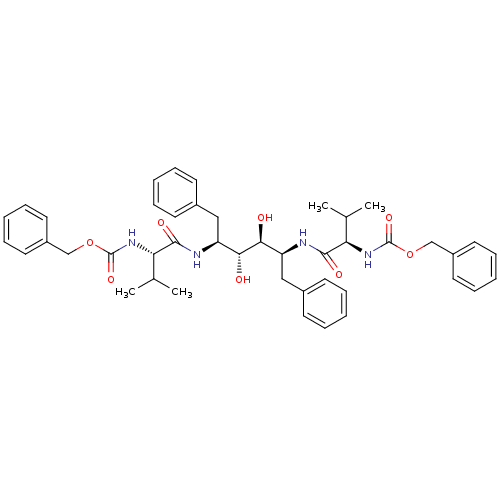 (CHEMBL314298 | {1-[1-Benzyl-4-(2-benzyloxycarbonyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against recombinant HIV-1 protease. | J Med Chem 33: 2687-9 (1990) BindingDB Entry DOI: 10.7270/Q2W37V89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50014149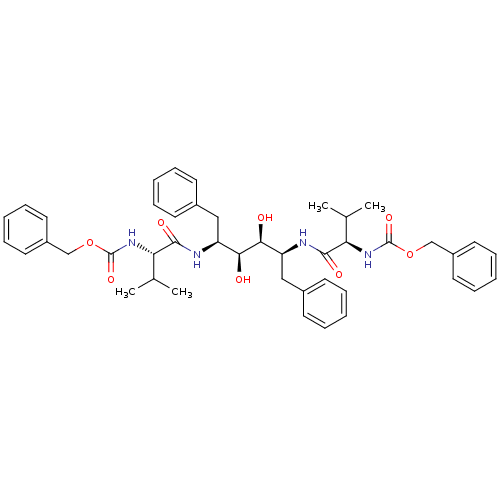 (CHEMBL314758 | {1-[1-Benzyl-4-(2-benzyloxycarbonyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against recombinant HIV-1 protease. | J Med Chem 33: 2687-9 (1990) BindingDB Entry DOI: 10.7270/Q2W37V89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50046401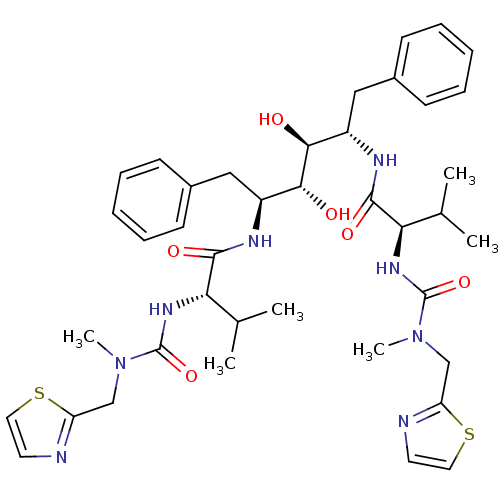 (CHEMBL432806 | N-{1-Benzyl-2,3-dihydroxy-4-[3-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of purified recombinant HIV-1 protease in a fluorogenic assay | J Med Chem 36: 320-30 (1993) BindingDB Entry DOI: 10.7270/Q25B01J6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50063071 (((1S,2S,4S)-1-Benzyl-2-hydroxy-4-{3-[3-(2-isopropy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description Inhibitory concentration against purified recombinant HIV-1 protease using fluorogenic substrate | J Med Chem 41: 602-17 (1998) Article DOI: 10.1021/jm970636+ BindingDB Entry DOI: 10.7270/Q2N58KGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50046439 (2N-[1-[1-benzyl-2-hydroxy-3-[1-(1H-2-indolylcarbox...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of purified recombinant HIV-1 protease in a fluorogenic assay | J Med Chem 36: 320-30 (1993) BindingDB Entry DOI: 10.7270/Q25B01J6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50062997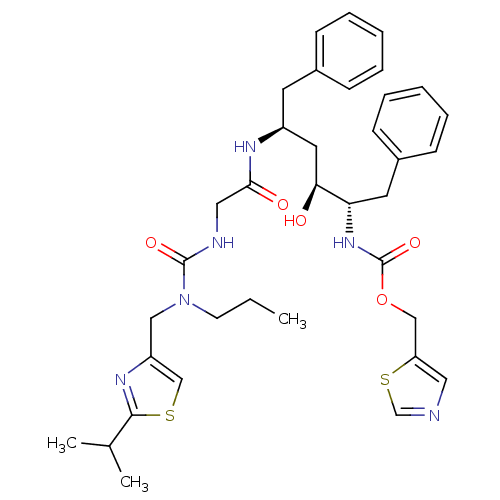 (((1S,2S,4S)-1-Benzyl-2-hydroxy-4-{2-[3-(2-isopropy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description Inhibitory concentration against purified recombinant HIV-1 protease using fluorogenic substrate | J Med Chem 41: 602-17 (1998) Article DOI: 10.1021/jm970636+ BindingDB Entry DOI: 10.7270/Q2N58KGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50174973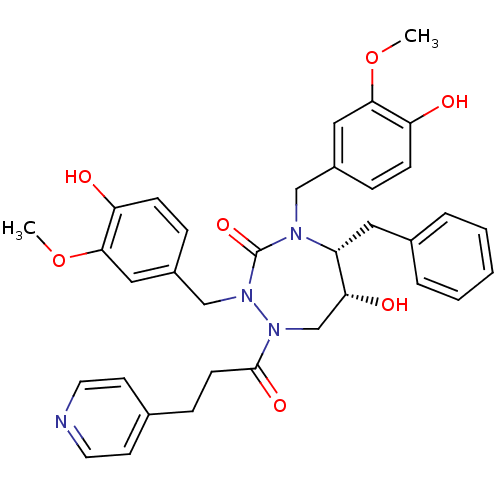 ((5R,6R)-2,4-bis(4-hydroxy-3-methoxybenzyl)-5-benzy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against HIV1 protease | Bioorg Med Chem Lett 15: 5499-503 (2005) Article DOI: 10.1016/j.bmcl.2005.08.093 BindingDB Entry DOI: 10.7270/Q2ZP45PR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50062946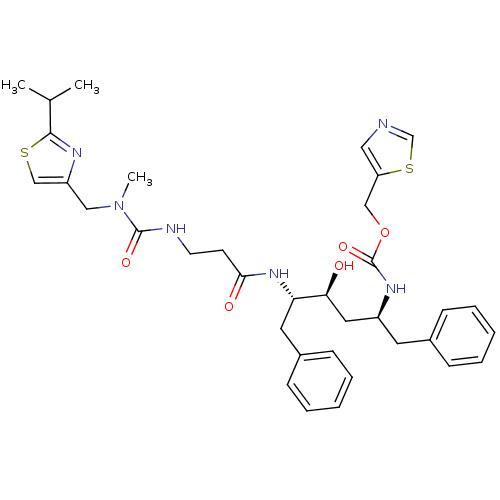 (((1S,3S,4S)-1-Benzyl-3-hydroxy-4-{3-[3-(2-isopropy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description Inhibitory concentration against purified recombinant HIV-1 protease using fluorogenic substrate | J Med Chem 41: 602-17 (1998) Article DOI: 10.1021/jm970636+ BindingDB Entry DOI: 10.7270/Q2N58KGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285916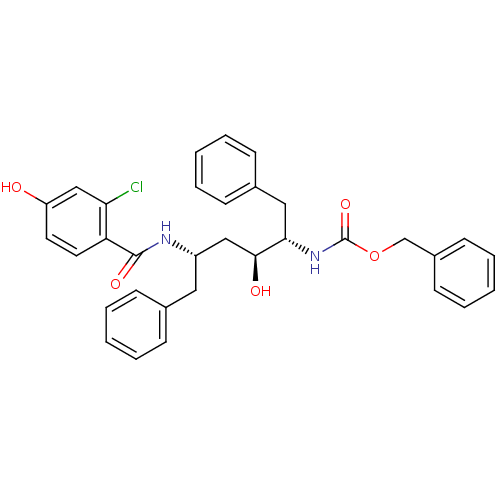 (CHEMBL90751 | [(1S,2S,4S)-1-Benzyl-4-(2-chloro-4-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against purified HIV protease | Bioorg Med Chem Lett 5: 2725-2728 (1995) Article DOI: 10.1016/0960-894X(95)00462-3 BindingDB Entry DOI: 10.7270/Q26973JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285903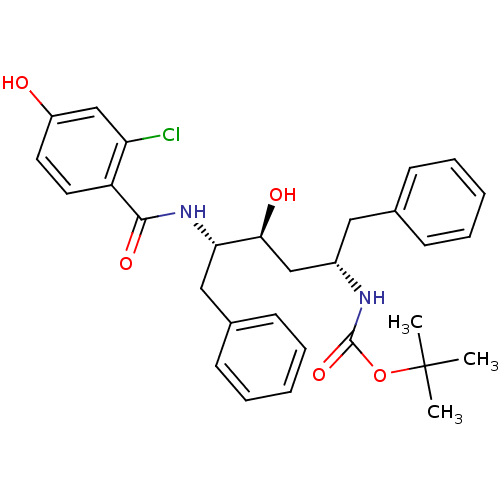 (CHEMBL93589 | [(1S,3S,4S)-1-Benzyl-4-(2-chloro-4-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against purified HIV protease | Bioorg Med Chem Lett 5: 2725-2728 (1995) Article DOI: 10.1016/0960-894X(95)00462-3 BindingDB Entry DOI: 10.7270/Q26973JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50046435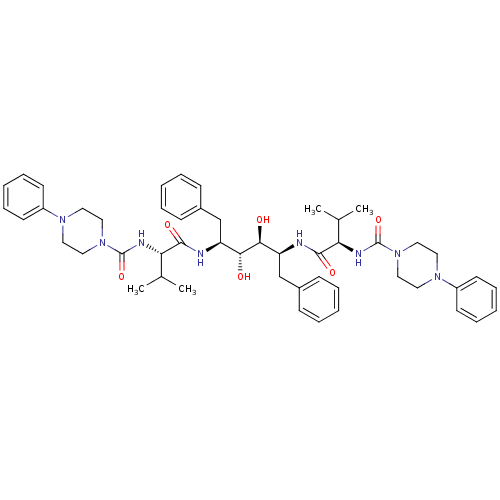 (1N-[1-[1-benzyl-2,3-dihydroxy-4-[2-methyl-1-(4-phe...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of purified recombinant HIV-1 protease in a fluorogenic assay | J Med Chem 36: 320-30 (1993) BindingDB Entry DOI: 10.7270/Q25B01J6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50046422 (CHEMBL324916 | N-{1-Benzyl-2,3-dihydroxy-4-[3-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of purified recombinant HIV-1 protease in a fluorogenic assay | J Med Chem 36: 320-30 (1993) BindingDB Entry DOI: 10.7270/Q25B01J6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50062994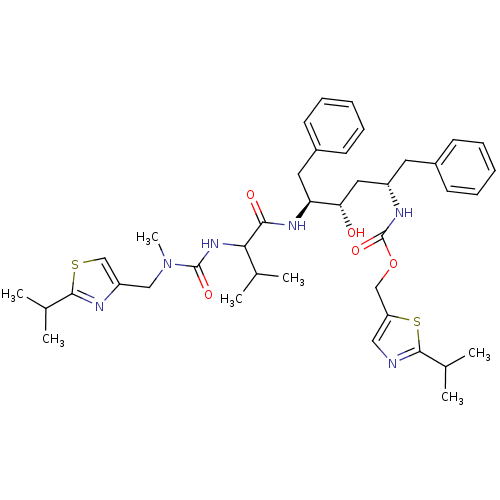 (((1S,3S,4S)-1-Benzyl-3-hydroxy-4-{2-[3-(2-isopropy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description Inhibitory concentration against purified recombinant HIV-1 protease using fluorogenic substrate | J Med Chem 41: 602-17 (1998) Article DOI: 10.1021/jm970636+ BindingDB Entry DOI: 10.7270/Q2N58KGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50046385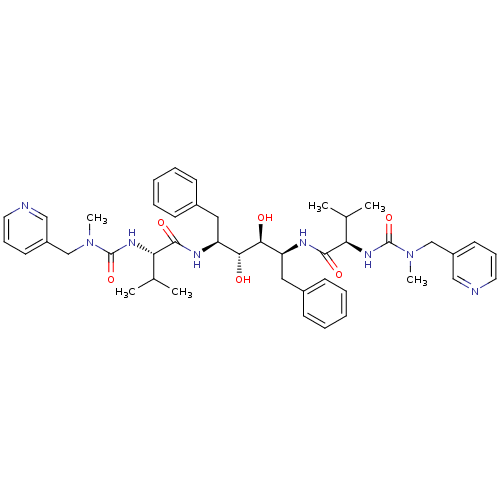 (CHEMBL331363 | N-{1-Benzyl-2,3-dihydroxy-4-[3-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of purified recombinant HIV-1 protease in a fluorogenic assay | J Med Chem 36: 320-30 (1993) BindingDB Entry DOI: 10.7270/Q25B01J6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50062954 (((1S,2S,4S)-1-Benzyl-2-hydroxy-4-{2-[3-isobutyl-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description Inhibitory concentration against purified recombinant HIV-1 protease using fluorogenic substrate | J Med Chem 41: 602-17 (1998) Article DOI: 10.1021/jm970636+ BindingDB Entry DOI: 10.7270/Q2N58KGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50046392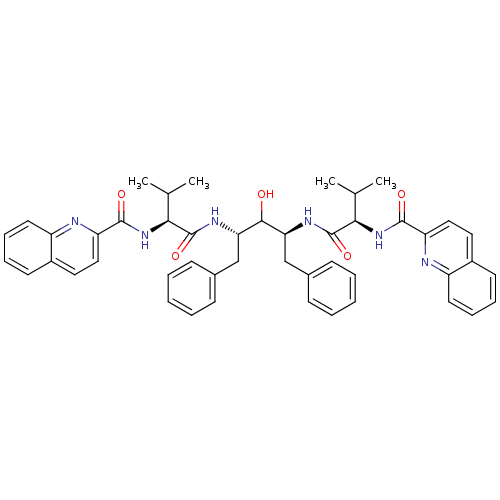 (2N-[1-[1-benzyl-2-hydroxy-3-[2-methyl-1-(2-quinoly...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of purified recombinant HIV-1 protease in a fluorogenic assay | J Med Chem 36: 320-30 (1993) BindingDB Entry DOI: 10.7270/Q25B01J6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50046428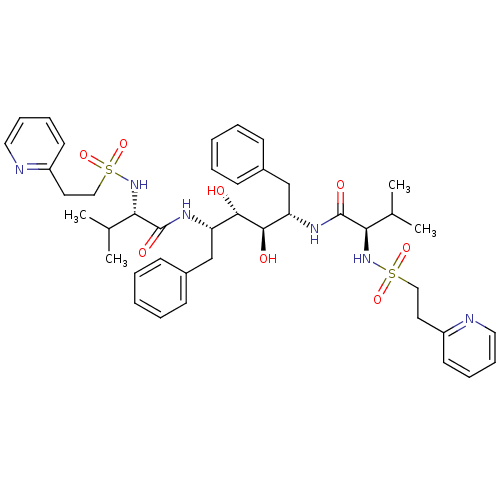 (CHEMBL112930 | N-{1-Benzyl-2,3-dihydroxy-4-[3-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of purified recombinant HIV-1 protease in a fluorogenic assay | J Med Chem 36: 320-30 (1993) BindingDB Entry DOI: 10.7270/Q25B01J6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285910 (CHEMBL93718 | [(1S,3S,4S)-1-Benzyl-3-hydroxy-4-(3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against purified HIV protease | Bioorg Med Chem Lett 5: 2725-2728 (1995) Article DOI: 10.1016/0960-894X(95)00462-3 BindingDB Entry DOI: 10.7270/Q26973JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50046384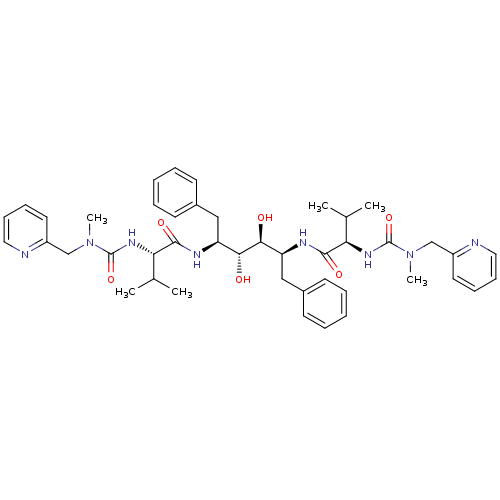 (CHEMBL113575 | N-{1-Benzyl-2,3-dihydroxy-4-[3-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.66 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of purified recombinant HIV-1 protease in a fluorogenic assay | J Med Chem 36: 320-30 (1993) BindingDB Entry DOI: 10.7270/Q25B01J6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50046408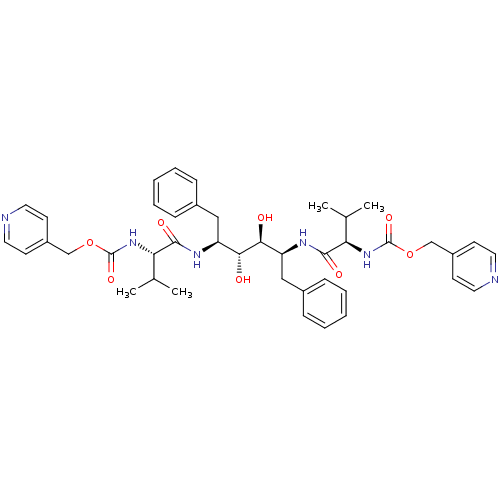 ((1-{1-Benzyl-2,3-dihydroxy-4-[3-methyl-2-(pyridin-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of purified recombinant HIV-1 protease in a fluorogenic assay | J Med Chem 36: 320-30 (1993) BindingDB Entry DOI: 10.7270/Q25B01J6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50062969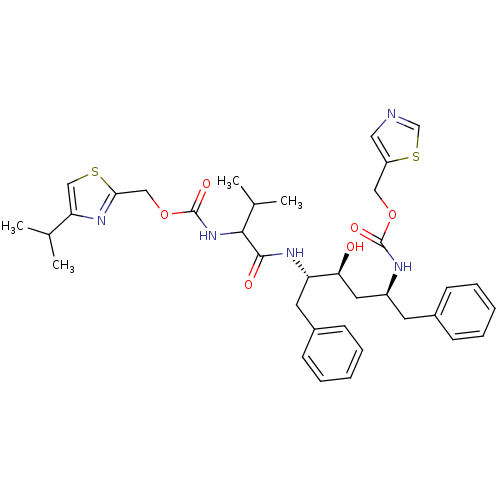 (CHEMBL346943 | {(1S,3S,4S)-1-Benzyl-3-hydroxy-4-[2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description Inhibitory concentration against purified recombinant HIV-1 protease using fluorogenic substrate | J Med Chem 41: 602-17 (1998) Article DOI: 10.1021/jm970636+ BindingDB Entry DOI: 10.7270/Q2N58KGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50062941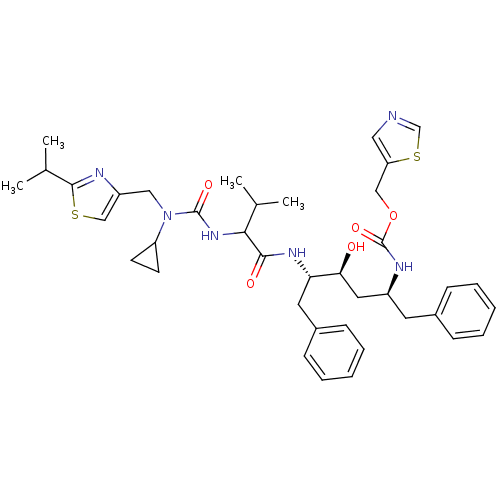 (((1S,3S,4S)-1-Benzyl-4-{2-[3-cyclopropyl-3-(2-isop...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description Inhibitory concentration against purified recombinant HIV-1 protease using fluorogenic substrate | J Med Chem 41: 602-17 (1998) Article DOI: 10.1021/jm970636+ BindingDB Entry DOI: 10.7270/Q2N58KGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50174993 ((5R,6R)-2,4-bis(4-hydroxy-3-methoxybenzyl)-5-benzy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against HIV1 protease | Bioorg Med Chem Lett 15: 5499-503 (2005) Article DOI: 10.1016/j.bmcl.2005.08.093 BindingDB Entry DOI: 10.7270/Q2ZP45PR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50046412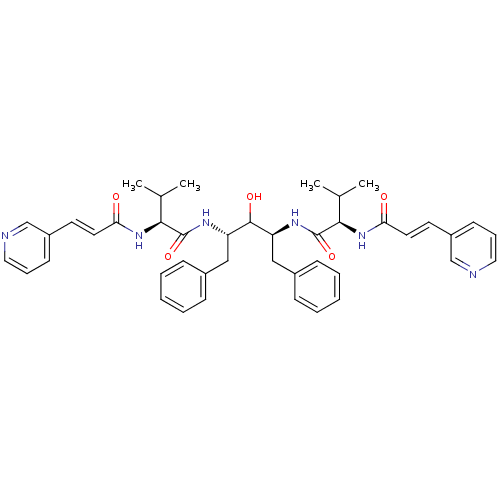 (CHEMBL113626 | N-{1-Benzyl-2-hydroxy-3-[3-methyl-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of purified recombinant HIV-1 protease in a fluorogenic assay | J Med Chem 36: 320-30 (1993) BindingDB Entry DOI: 10.7270/Q25B01J6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50062939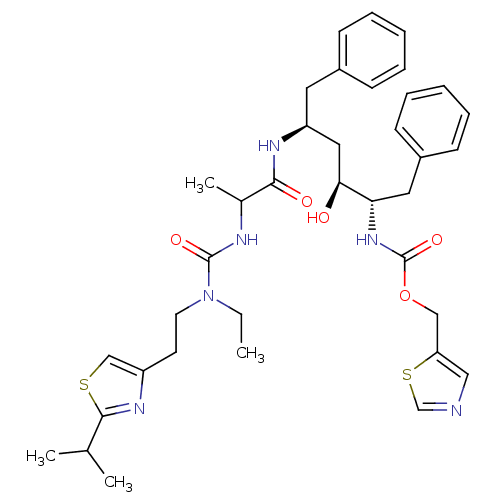 (CHEMBL347824 | [(1S,2S,4S)-1-Benzyl-4-(2-{3-ethyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description Inhibitory concentration against purified recombinant HIV-1 protease using fluorogenic substrate | J Med Chem 41: 602-17 (1998) Article DOI: 10.1021/jm970636+ BindingDB Entry DOI: 10.7270/Q2N58KGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50046390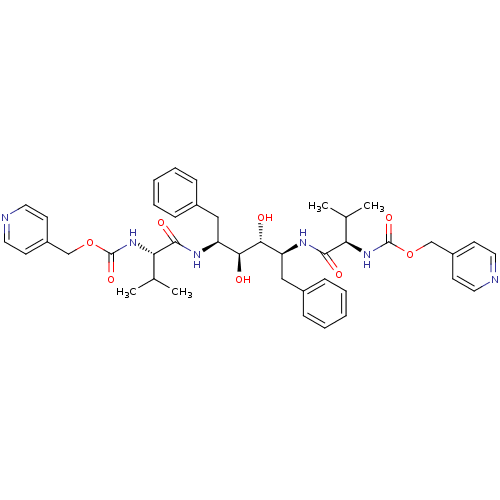 ((1-{1-Benzyl-2,3-dihydroxy-4-[3-methyl-2-(pyridin-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of purified recombinant HIV-1 protease in a fluorogenic assay | J Med Chem 36: 320-30 (1993) BindingDB Entry DOI: 10.7270/Q25B01J6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285911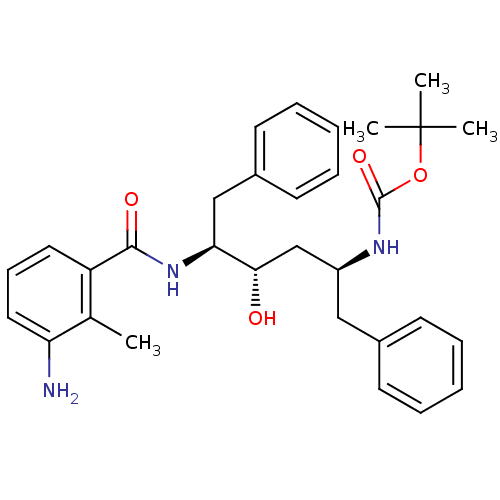 (CHEMBL90090 | [(1S,3S,4S)-4-(3-Amino-2-methyl-benz...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against purified HIV protease | Bioorg Med Chem Lett 5: 2725-2728 (1995) Article DOI: 10.1016/0960-894X(95)00462-3 BindingDB Entry DOI: 10.7270/Q26973JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50014147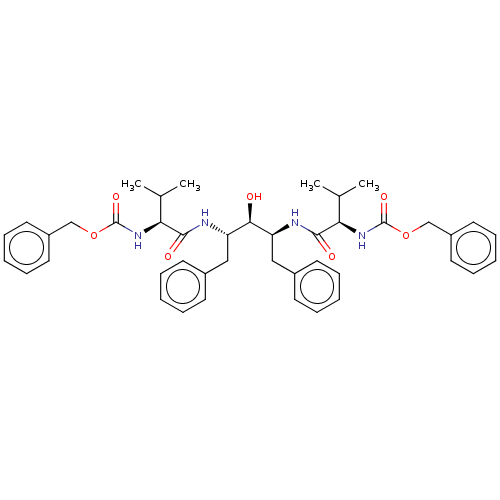 (A-74704 | CHEMBL430891 | {(S)-1-[(1S,3S)-1-Benzyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of purified recombinant HIV-1 protease in a fluorogenic assay | J Med Chem 36: 320-30 (1993) BindingDB Entry DOI: 10.7270/Q25B01J6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50046378 (CHEMBL109371 | {1-[1-Benzyl-3-(2-benzyloxycarbonyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of purified recombinant HIV-1 protease in a fluorogenic assay | J Med Chem 36: 320-30 (1993) BindingDB Entry DOI: 10.7270/Q25B01J6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50014147 (A-74704 | CHEMBL430891 | {(S)-1-[(1S,3S)-1-Benzyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against recombinant HIV-1 Protease | J Med Chem 33: 2687-9 (1990) BindingDB Entry DOI: 10.7270/Q2W37V89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285913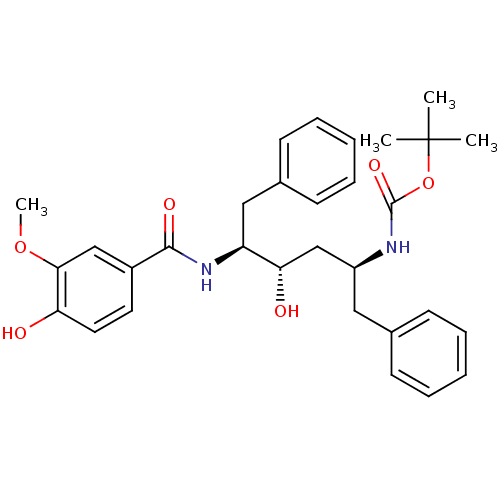 (CHEMBL327611 | [(1S,3S,4S)-1-Benzyl-3-hydroxy-4-(4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against purified HIV protease | Bioorg Med Chem Lett 5: 2725-2728 (1995) Article DOI: 10.1016/0960-894X(95)00462-3 BindingDB Entry DOI: 10.7270/Q26973JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285890 (CHEMBL93566 | [(1S,3S,4S)-4-(5-Amino-2-chloro-benz...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against purified HIV protease | Bioorg Med Chem Lett 5: 2725-2728 (1995) Article DOI: 10.1016/0960-894X(95)00462-3 BindingDB Entry DOI: 10.7270/Q26973JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285905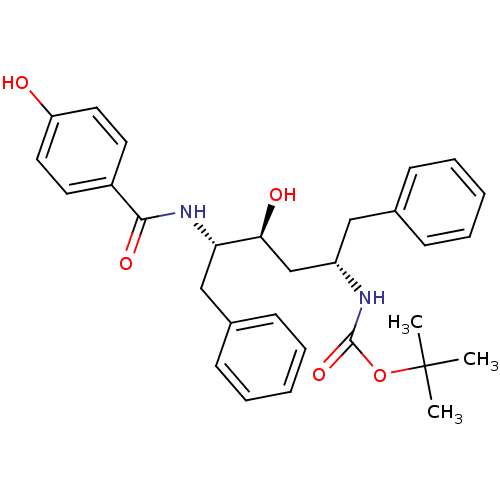 (CHEMBL93540 | [(1S,3S,4S)-1-Benzyl-3-hydroxy-4-(4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against purified HIV protease | Bioorg Med Chem Lett 5: 2725-2728 (1995) Article DOI: 10.1016/0960-894X(95)00462-3 BindingDB Entry DOI: 10.7270/Q26973JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285897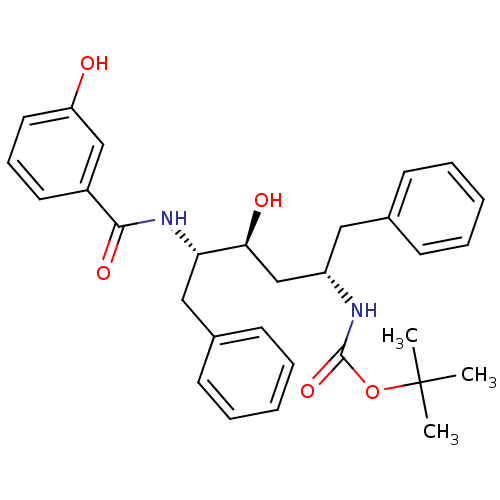 (CHEMBL273562 | [(1S,3S,4S)-1-Benzyl-3-hydroxy-4-(3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against purified HIV protease | Bioorg Med Chem Lett 5: 2725-2728 (1995) Article DOI: 10.1016/0960-894X(95)00462-3 BindingDB Entry DOI: 10.7270/Q26973JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 128 total ) | Next | Last >> |