| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Reverse transcriptase/RNaseH |
|---|
| Ligand | BDBM33411 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_2204627 (CHEMBL5117335) |
|---|
| IC50 | 1980±n/a nM |
|---|
| Citation |  Zhang, L; Wei, F; Borrego, D; Zhao, F; Río, JMD; Frutos-Beltrán, E; Zhang, J; Xu, S; López-Carrobles, N; Gao, S; Kang, D; Pannecouque, C; Clercq, E; Liu, X; Menéndez-Arias, L; Zhan, P Design, synthesis, and biological evaluation of novel double-winged galloyl derivatives as HIV-1 RNase H inhibitors. Eur J Med Chem240:0 (2022) [PubMed] Article Zhang, L; Wei, F; Borrego, D; Zhao, F; Río, JMD; Frutos-Beltrán, E; Zhang, J; Xu, S; López-Carrobles, N; Gao, S; Kang, D; Pannecouque, C; Clercq, E; Liu, X; Menéndez-Arias, L; Zhan, P Design, synthesis, and biological evaluation of novel double-winged galloyl derivatives as HIV-1 RNase H inhibitors. Eur J Med Chem240:0 (2022) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Reverse transcriptase/RNaseH |
|---|
| Name: | Reverse transcriptase/RNaseH |
|---|
| Synonyms: | HIV-1 Reverse Transcriptase RNase H | Human immunodeficiency virus type 1 reverse transcriptase | Reverse transcriptase/RNaseH |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 65229.15 |
|---|
| Organism: | Human immunodeficiency virus 1 |
|---|
| Description: | ChEMBL_1473730 |
|---|
| Residue: | 566 |
|---|
| Sequence: | PISPIETVPVKLKPGMDGPKVKQWPLTEEKIKALVEICTEMEKEGKISKIGPENPYNTPV
FAIKKKDSTKWRKLVDFRELNKRTQDFWEVQLGIPHPAGLKKRKSVTVLDVGDAYFSVPL
DEDFRKYTAFTIPSINNETPGIRYQYNVLPQGWKGSPAIFQSSMTKILEPFRKQNPDIVI
YQYMDDLYVGSDLEIGQHRTKIEELRQHLLRWGLTTPDKKHQKEPPFLWMGYELHPDKWT
VQPIVLPEKDSWTVNDIQKLVGKLNWASQIYPGIRVRQLCKLLRGTKALTEVIPLTEEAE
LELAENREILKEPVHGVYYDPSKDLIAEIQKQGQGQWTYQIYQEPFKNLRTGKYARMRGA
HTNDVKQLTEAVQKITTESIVIWGKTPKFKLPIQKETWETWWTEYWQATWIPEWEFVNTP
PLVKLWYQLEKEPIVGAETFYVDGAANRETKLGKAGYVTNRGRQKVVTLTDTTNQKTELQ
AIYLALQDSGLEVNIVTDSQYALGIIQAQPDQSESELVNQIIEQLIKKEKVYLAWVPAHK
GIGGNEQVDKLVSAGIRKVLFLDGID
|
|
|
|---|
| BDBM33411 |
|---|
| n/a |
|---|
| Name | BDBM33411 |
|---|
| Synonyms: | β-Thujaplicinol | hydroxytropolone, 3 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C10H12O3 |
|---|
| Mol. Mass. | 180.2005 |
|---|
| SMILES | CC(C)c1ccc(O)c(=O)c(O)c1 |
|---|
| Structure | 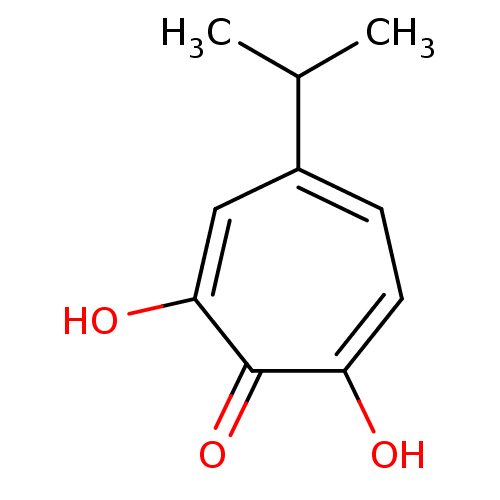
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Zhang, L; Wei, F; Borrego, D; Zhao, F; Río, JMD; Frutos-Beltrán, E; Zhang, J; Xu, S; López-Carrobles, N; Gao, S; Kang, D; Pannecouque, C; Clercq, E; Liu, X; Menéndez-Arias, L; Zhan, P Design, synthesis, and biological evaluation of novel double-winged galloyl derivatives as HIV-1 RNase H inhibitors. Eur J Med Chem240:0 (2022) [PubMed] Article
Zhang, L; Wei, F; Borrego, D; Zhao, F; Río, JMD; Frutos-Beltrán, E; Zhang, J; Xu, S; López-Carrobles, N; Gao, S; Kang, D; Pannecouque, C; Clercq, E; Liu, X; Menéndez-Arias, L; Zhan, P Design, synthesis, and biological evaluation of novel double-winged galloyl derivatives as HIV-1 RNase H inhibitors. Eur J Med Chem240:0 (2022) [PubMed] Article