| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Glutamate receptor 2 |
|---|
| Ligand | BDBM50192226 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_391778 (CHEMBL871565) |
|---|
| EC50 | >3000±n/a nM |
|---|
| Citation |  Fernandez, MC; Castaño, A; Dominguez, E; Escribano, A; Jiang, D; Jimenez, A; Hong, E; Hornback, WJ; Nisenbaum, ES; Rankl, N; Tromiczak, E; Vaught, G; Zarrinmayeh, H; Zimmerman, DM A novel class of AMPA receptor allosteric modulators. Part 1: design, synthesis, and SAR of 3-aryl-4-cyano-5-substituted-heteroaryl-2-carboxylic acid derivatives. Bioorg Med Chem Lett16:5057-61 (2006) [PubMed] Article Fernandez, MC; Castaño, A; Dominguez, E; Escribano, A; Jiang, D; Jimenez, A; Hong, E; Hornback, WJ; Nisenbaum, ES; Rankl, N; Tromiczak, E; Vaught, G; Zarrinmayeh, H; Zimmerman, DM A novel class of AMPA receptor allosteric modulators. Part 1: design, synthesis, and SAR of 3-aryl-4-cyano-5-substituted-heteroaryl-2-carboxylic acid derivatives. Bioorg Med Chem Lett16:5057-61 (2006) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Glutamate receptor 2 |
|---|
| Name: | Glutamate receptor 2 |
|---|
| Synonyms: | AMPA-selective glutamate receptor 2 | GLUR2 | GRIA2 | GRIA2_HUMAN | GluR-2 | GluR-B | GluR-K2 | Glutamate AMPA 2 | Glutamate receptor 2 | Glutamate receptor AMPA 1/2 | Glutamate receptor AMPA 2/3 | Glutamate receptor ionotropic AMPA | Glutamate receptor ionotropic, AMPA 2 |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 98825.96 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Glutamate AMPA 2 GRIA2 HUMAN::P42262 |
|---|
| Residue: | 883 |
|---|
| Sequence: | MQKIMHISVLLSPVLWGLIFGVSSNSIQIGGLFPRGADQEYSAFRVGMVQFSTSEFRLTP
HIDNLEVANSFAVTNAFCSQFSRGVYAIFGFYDKKSVNTITSFCGTLHVSFITPSFPTDG
THPFVIQMRPDLKGALLSLIEYYQWDKFAYLYDSDRGLSTLQAVLDSAAEKKWQVTAINV
GNINNDKKDEMYRSLFQDLELKKERRVILDCERDKVNDIVDQVITIGKHVKGYHYIIANL
GFTDGDLLKIQFGGANVSGFQIVDYDDSLVSKFIERWSTLEEKEYPGAHTTTIKYTSALT
YDAVQVMTEAFRNLRKQRIEISRRGNAGDCLANPAVPWGQGVEIERALKQVQVEGLSGNI
KFDQNGKRINYTINIMELKTNGPRKIGYWSEVDKMVVTLTELPSGNDTSGLENKTVVVTT
ILESPYVMMKKNHEMLEGNERYEGYCVDLAAEIAKHCGFKYKLTIVGDGKYGARDADTKI
WNGMVGELVYGKADIAIAPLTITLVREEVIDFSKPFMSLGISIMIKKPQKSKPGVFSFLD
PLAYEIWMCIVFAYIGVSVVLFLVSRFSPYEWHTEEFEDGRETQSSESTNEFGIFNSLWF
SLGAFMQQGCDISPRSLSGRIVGGVWWFFTLIIISSYTANLAAFLTVERMVSPIESAEDL
SKQTEIAYGTLDSGSTKEFFRRSKIAVFDKMWTYMRSAEPSVFVRTTAEGVARVRKSKGK
YAYLLESTMNEYIEQRKPCDTMKVGGNLDSKGYGIATPKGSSLRNAVNLAVLKLNEQGLL
DKLKNKWWYDKGECGSGGGDSKEKTSALSLSNVAGVFYILVGGLGLAMLVALIEFCYKSR
AEAKRMKVAKNAQNINPSSSQNSQNFATYKEGYNVYGIESVKI
|
|
|
|---|
| BDBM50192226 |
|---|
| n/a |
|---|
| Name | BDBM50192226 |
|---|
| Synonyms: | 3-(4-tert-butylphenyl)-4-cyanothiophene-2-carboxylic acid | CHEMBL385502 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C16H15NO2S |
|---|
| Mol. Mass. | 285.361 |
|---|
| SMILES | CC(C)(C)c1ccc(cc1)-c1c(csc1C(O)=O)C#N |
|---|
| Structure | 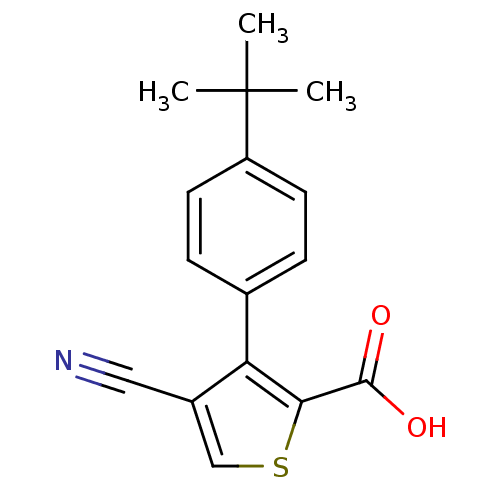
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Fernandez, MC; Castaño, A; Dominguez, E; Escribano, A; Jiang, D; Jimenez, A; Hong, E; Hornback, WJ; Nisenbaum, ES; Rankl, N; Tromiczak, E; Vaught, G; Zarrinmayeh, H; Zimmerman, DM A novel class of AMPA receptor allosteric modulators. Part 1: design, synthesis, and SAR of 3-aryl-4-cyano-5-substituted-heteroaryl-2-carboxylic acid derivatives. Bioorg Med Chem Lett16:5057-61 (2006) [PubMed] Article
Fernandez, MC; Castaño, A; Dominguez, E; Escribano, A; Jiang, D; Jimenez, A; Hong, E; Hornback, WJ; Nisenbaum, ES; Rankl, N; Tromiczak, E; Vaught, G; Zarrinmayeh, H; Zimmerman, DM A novel class of AMPA receptor allosteric modulators. Part 1: design, synthesis, and SAR of 3-aryl-4-cyano-5-substituted-heteroaryl-2-carboxylic acid derivatives. Bioorg Med Chem Lett16:5057-61 (2006) [PubMed] Article