

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50130563 ((3R)-7-Hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-hydroxyphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0591 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid kappa receptor expressed in CHO cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.153 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid kappa receptor expressed in CHO cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50026614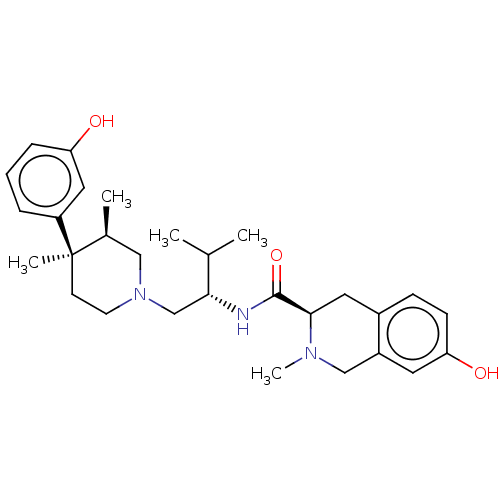 (CHEMBL575508) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.153 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid kappa receptor expressed in CHO cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50346474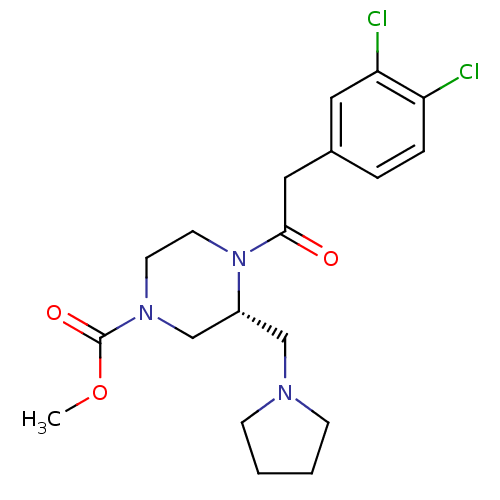 ((R)-4-[2-(3,4-Dichloro-phenyl)-acetyl]-3-pyrrolidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.502 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid kappa receptor expressed in CHO cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50358169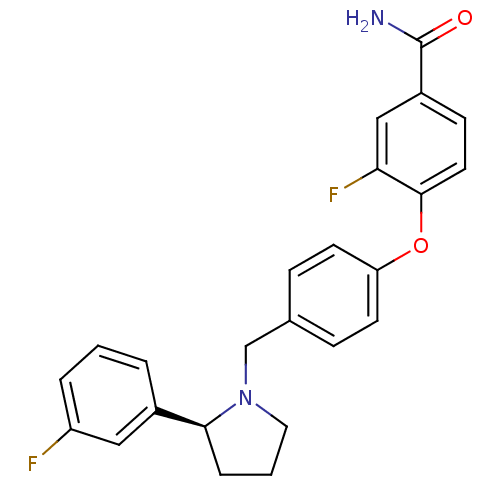 (CHEMBL1921845) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.565 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid kappa receptor expressed in CHO cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50358168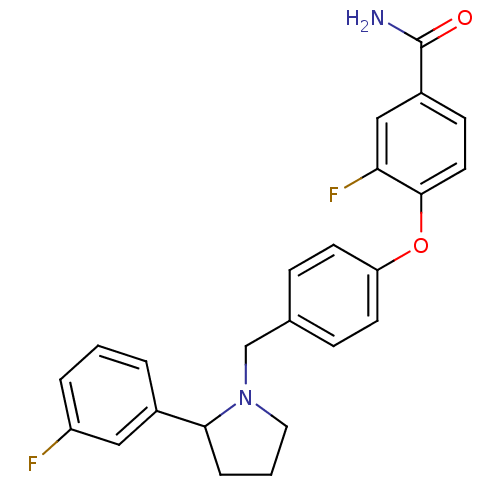 (CHEMBL1921844) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.617 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid kappa receptor expressed in CHO cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50358165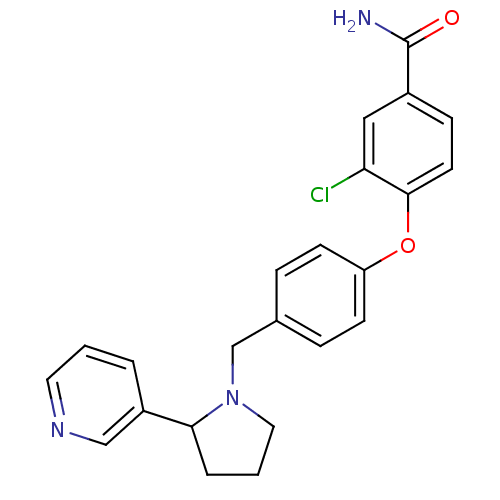 (CHEMBL1921841) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.622 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid kappa receptor expressed in CHO cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50358166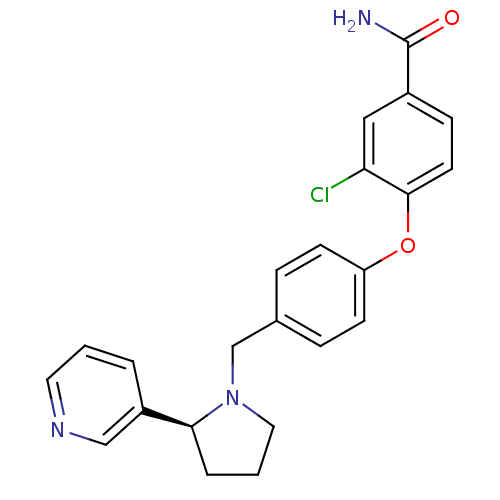 (CHEMBL1921842) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.722 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid kappa receptor expressed in CHO cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50358171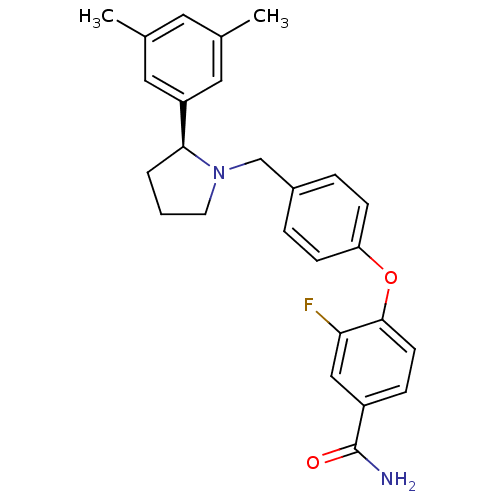 (CHEMBL1921847) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.949 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid kappa receptor expressed in CHO cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50358161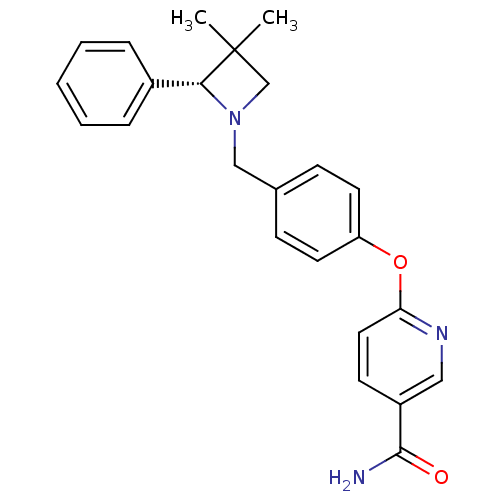 (CHEMBL1921850) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid kappa receptor expressed in CHO cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50358160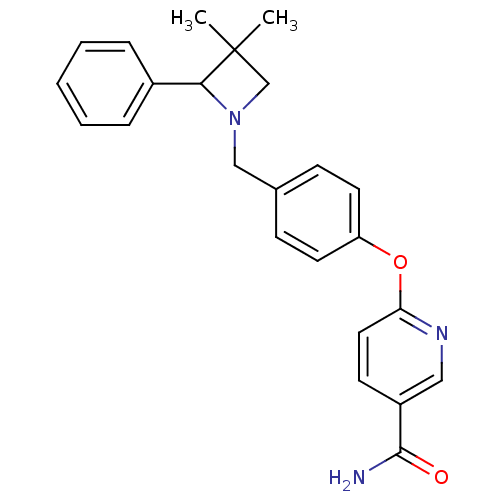 (CHEMBL1921849) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid kappa receptor expressed in CHO cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50358164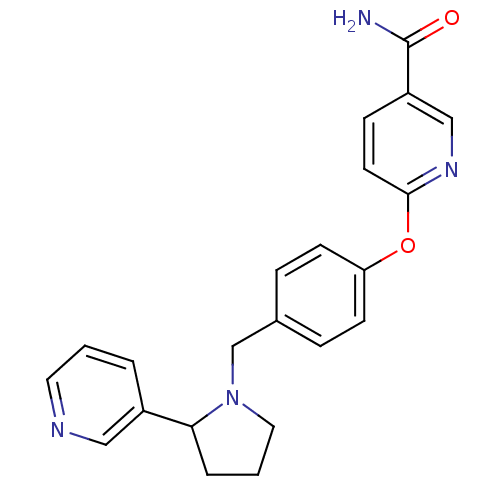 (CHEMBL1921840) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid kappa receptor expressed in CHO cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50219916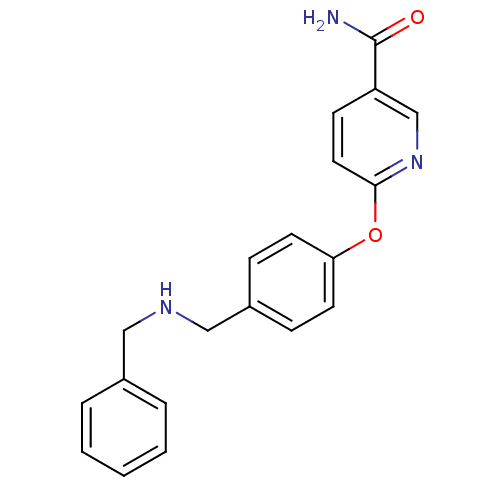 (6-(4-((benzylamino)methyl)phenoxy)nicotinamide | C...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid kappa receptor expressed in CHO cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50219916 (6-(4-((benzylamino)methyl)phenoxy)nicotinamide | C...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid mu receptor expressed in CHO cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid delta receptor expressed in HEK293 cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50358163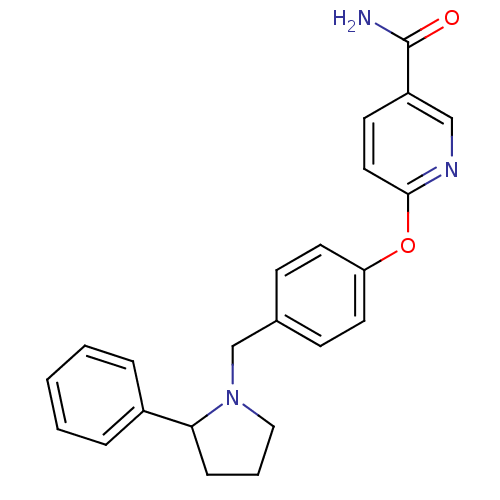 (CHEMBL1921839) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid kappa receptor expressed in CHO cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50130563 ((3R)-7-Hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-hydroxyphe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid mu receptor expressed in CHO cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50358172 (CHEMBL1921848) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid kappa receptor expressed in CHO cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50358171 (CHEMBL1921847) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid mu receptor expressed in CHO cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50358166 (CHEMBL1921842) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid mu receptor expressed in CHO cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50358162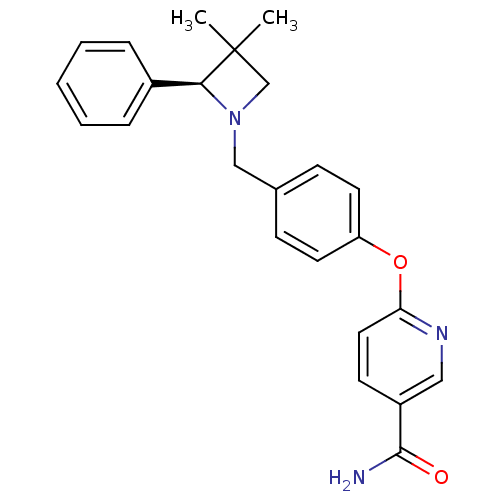 (CHEMBL1921851) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid kappa receptor expressed in CHO cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50219916 (6-(4-((benzylamino)methyl)phenoxy)nicotinamide | C...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid delta receptor expressed in HEK293 cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Syntenin-1 (Homo sapiens) | BDBM50557298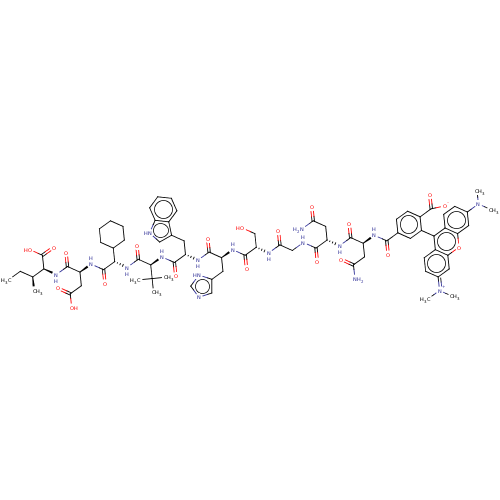 (CHEMBL4750927) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of syntenin-PDZ1-2 domain (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 5 mins by fluorescence polarization assa... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00382 BindingDB Entry DOI: 10.7270/Q2V98CRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50026614 (CHEMBL575508) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid mu receptor expressed in CHO cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50358165 (CHEMBL1921841) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid mu receptor expressed in CHO cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid mu receptor expressed in CHO cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50346474 ((R)-4-[2-(3,4-Dichloro-phenyl)-acetyl]-3-pyrrolidi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid mu receptor expressed in CHO cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50358169 (CHEMBL1921845) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid mu receptor expressed in CHO cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Syntenin-1 (Homo sapiens) | BDBM50557293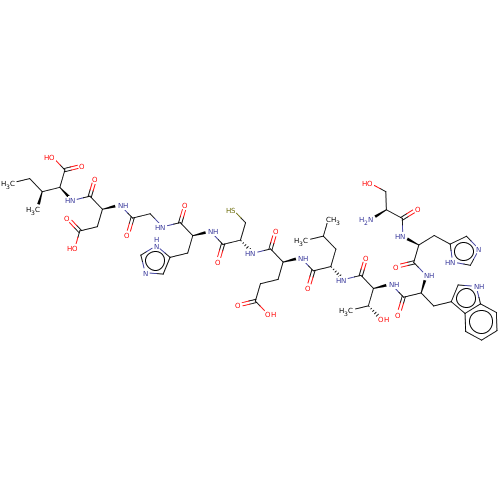 (CHEMBL4781637) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of syntenin-PDZ1-2 domain (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 5 mins by fluorescence polarization assa... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00382 BindingDB Entry DOI: 10.7270/Q2V98CRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50358164 (CHEMBL1921840) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid mu receptor expressed in CHO cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50358168 (CHEMBL1921844) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid mu receptor expressed in CHO cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50358161 (CHEMBL1921850) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid mu receptor expressed in CHO cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50358167 (CHEMBL1921843) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid kappa receptor expressed in CHO cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50358160 (CHEMBL1921849) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid mu receptor expressed in CHO cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50358170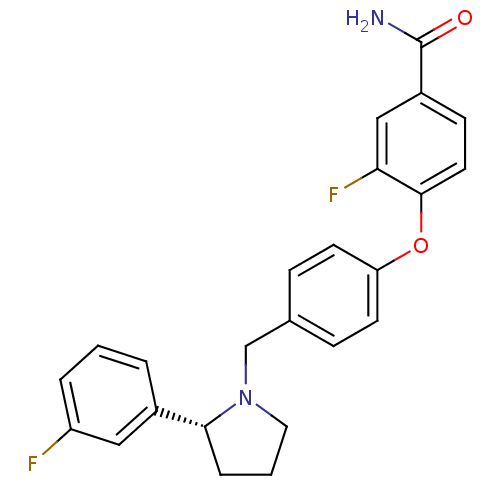 (CHEMBL1921846) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid kappa receptor expressed in CHO cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50358172 (CHEMBL1921848) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid mu receptor expressed in CHO cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50358162 (CHEMBL1921851) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid mu receptor expressed in CHO cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50358166 (CHEMBL1921842) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 153 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid delta receptor expressed in HEK293 cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50358172 (CHEMBL1921848) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 165 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid delta receptor expressed in HEK293 cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50358171 (CHEMBL1921847) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 166 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid delta receptor expressed in HEK293 cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Syntenin-1 (Homo sapiens) | BDBM50557299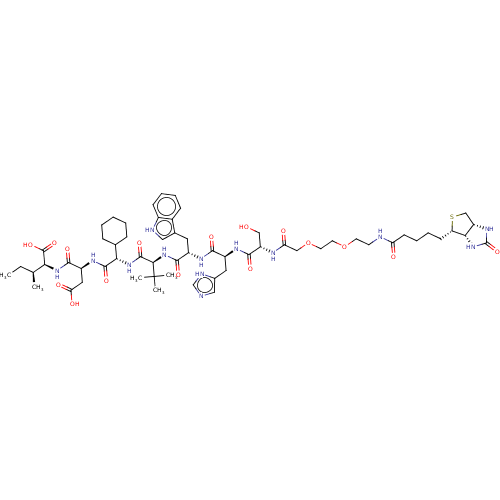 (CHEMBL4799812) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to syntenin-PDZ1-2 domain (unknown origin) expressed in Escherichia coli BL21 (DE3) by SPR analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00382 BindingDB Entry DOI: 10.7270/Q2V98CRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50358168 (CHEMBL1921844) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 187 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid delta receptor expressed in HEK293 cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50130563 ((3R)-7-Hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-hydroxyphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 188 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid delta receptor expressed in HEK293 cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Syntenin-1 (Homo sapiens) | BDBM50557299 (CHEMBL4799812) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of syntenin-PDZ1-2 domain (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 5 mins by fluorescence polarization assa... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00382 BindingDB Entry DOI: 10.7270/Q2V98CRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50358169 (CHEMBL1921845) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 211 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid delta receptor expressed in HEK293 cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50358163 (CHEMBL1921839) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 232 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid mu receptor expressed in CHO cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50213115 ((R,R)-(-)-5-{1-hydroxy-2-[1-methyl-2-(1-naphthyl)e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 241 | -35.1 | n/a | n/a | n/a | n/a | n/a | 7.8 | 4 |
The United States of America, as represented by the Secretary, Department of Health and Human Services; SRI International US Patent | Assay Description Beta1-AR binding was done on rat cortical membrane following a previously described procedure (Beer et al., Biochem. Pharmacol. 37: 1145-1151, 1988).... | US Patent US9492405 (2016) BindingDB Entry DOI: 10.7270/Q2CJ8CBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Syntenin-1 (Homo sapiens) | BDBM50557297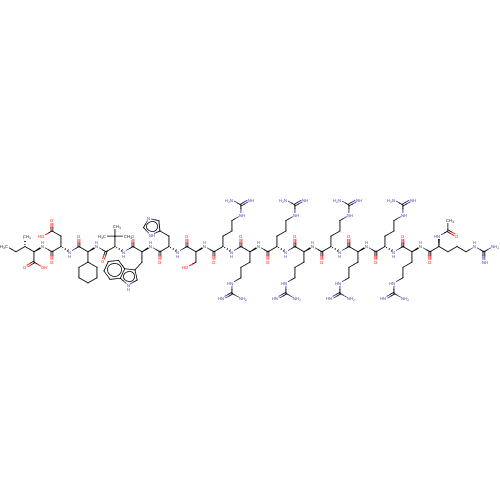 (CHEMBL4747962) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of syntenin-PDZ1-2 domain (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 5 mins by fluorescence polarization assa... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00382 BindingDB Entry DOI: 10.7270/Q2V98CRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Syntenin-1 (Homo sapiens) | BDBM50557296 (CHEMBL4792861) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of syntenin-PDZ1-2 domain (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 5 mins by fluorescence polarization assa... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00382 BindingDB Entry DOI: 10.7270/Q2V98CRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Syntenin-1 (Homo sapiens) | BDBM50557295 (CHEMBL4758599) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of syntenin-PDZ1-2 domain (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 5 mins by fluorescence polarization assa... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00382 BindingDB Entry DOI: 10.7270/Q2V98CRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 490 total ) | Next | Last >> |